How does grass grow in the extremely hot soils of Yellowstone National Park? Could a protein from a virus help plants handle global warming? Okay, that second sentence is wild speculation, but we will try to find the answer to our mystery by aligning our protein sequence to a sequence from a related structure.
tags: plants, ![]() bioinformatics, sequence analysis, viruses, fungi,
bioinformatics, sequence analysis, viruses, fungi, ![]() global warming,
global warming,
Read part I, part II, part III, part IV, and part V, to see how we got here.
This week, in our last installment, we will seek the answers in a related structure.
Last week, I found that my unidentified viral protein was related to a protein in a structure called 2CLB. This week, I'm hot on the trail.
From the Related Structures page, I clicked the pink bar graph, showing where the Aspergillus protein aligns to the protein from 2CLB, chain A (I could have used any of the eight chains, they're all identical).
That took me to a page with a Get 3D Structure data button.
Who can resist something like that?
I downloaded the 2CLB structure with the Aspergillus sequence, already aligned and opened the file in Cn3D.
Now, I needed to see how my 331 amino acid protein from the Curvularia virus aligned with the structure. I imported the Curvularia sequence and used the algorithms in Cn3D to create the alignment, making sure that the alignment between the Curvularia sequence and the Aspergillus sequence was still correct.
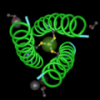
After aligning the sequences, I changed the color to show variety. Red letters, like the tryptophan (W) are the most important.
But how conserved is the function?
I noticed that this protein binds both iron and zinc so I annotated the structure to learn which amino acids contact the iron and the zinc. It would have been ideal if the amino acids at the equivalent positions in the Aspergillus and Curvularia proteins had been the same residues as those in the 2CLB protein. Unfortunately, they're not. So, I don't know if the fungal proteins share the metal binding property that we see in 2CLB or they only share the helical shape.
This picture below, shows the amino acids that align in either red or blue. Red amino acids are identical. Blue amino acids are not.

Now, I suppose you're wondering just what it is that we know about the protein in 2CLB. The protein in 2CLB is a DPS-like protein from Sulfolobus solfactaricus. These archea are hyperthermophilic acidophiles, meaning that they like a hot, acid environment.
(Remember, we're trying find out how our grass tolerates a high temperature).
And, better yet, the protein DPS (short for DNA-binding protein from starved cells) may play a role in protecting DNA from oxidative stress. So, to find a similar protein in bacteria that live at high temperatures could be an important clue.
At end of the day, just like Kansas City, we've gone about as fur as we can go.
To summarize the results at this point, we know:
- Part of the viral protein has a structure that's similar to the structure of 2CLB
- The 2CLB structure binds metal ions, but the viral protein might not.
Time for the wet lab
Now it's time for wet lab experiments. If I were working on this, I would see if growing the grass in iron makes a difference. I would also try to make some transgenic plants and see if the 331 amino acid protein alone, can confer the ability to withstand heat.
But all that work requires a lab, so at the end of the day, I guess I'll just put in an alert at PubMed and follow the story from my computer.
References:
1. Márquez, L., et. al. 2007 A Virus in a Fungus in a Plant: Three-Way Symbiosis Required for Thermal Tolerance Science 26: 513-515.
2. Wang Y, Addess KJ, Chen J, Geer LY, He J, He S, Lu S, Madej T, Marchler-Bauer A, Thiessen PA, Zhang N, Bryant SH. 2007. MMDB: annotating protein sequences with Entrez's 3D-structure database. Nucleic Acids Res. 2007 Jan;35(Database issue):D298-300.
3. George H. Gauss, Philippe Benas, Blake Wiedenheft, Mark Young, Trevor, Douglas, and C. Martin Lawrence 2006. Structure of the DPS-Like Protein from Sulfolobus solfataricus Reveals a Bacterioferritin-Like Dimetal Binding Site within a DPS-Like Dodecameric Assembly. Biochemistry 45(36): 10815-10827.
- Log in to post comments