Image modified.
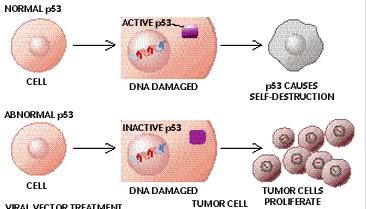
When I was an undergrad, I worked in a lab that studied p53, a gene that acts as a tumor suppressor. At that time, it was thought that most cancers resulted from a defective form of p53 or after p53 had inadvertently been turned off because p53 controls the cycle of cell proliferation, telling the cell when it is appropriate to divide, activating the cell's DNA repair mechanisms and preventing cells with damaged DNA from dividing. At that time, it was thought that cancerous tumors could be treated and possibly cured by reactivating p53 or by injecting an undamaged copy of p53 into an existing tumor.
Recently, a paper was published in the top-tier peer-reviewed journal, Nature, that describes the results of a series of studies in a special type of mouse that had been developed to have an inactive form of p53. This special mouse was developed to have a "molecular switch" in the animal's DNA such that researchers could activate p53 at will in the living animal.
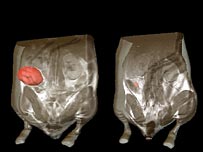 Using this mouse model, two teams of researchers discovered that those mice with deactivated p53 gene developed tumors, but that after the researchers activated p53, even briefly, the tumors regressed, sometimes by 100% (pictured, the tumor is in pink).
Using this mouse model, two teams of researchers discovered that those mice with deactivated p53 gene developed tumors, but that after the researchers activated p53, even briefly, the tumors regressed, sometimes by 100% (pictured, the tumor is in pink).
One group was from New York's Cold Spring Harbor Laboratory and the other was from Massachusetts Institute of Technology (MIT) in Cambridge.
Curiously, the MIT team also found that once activated, p53 caused different effects in different cancers: it caused lymphoma cells to undergo apoptosis (programmed cell death) while the Cold Spring Harbor team found that sarcoma calls simply aged and lost their ability to divide. Lymphomas are cancers caused by lymphocytes, a blood cell that is part of the body's immune system, whereas sarcomas are cancers of the connective tissue, such as bone or muscle.
The research teams are not sure why these two cancers are affected in different ways, but have started identifying those genes that are activated in each type of tumor when p53 turns on again.
"This study provides critical genetic evidence that continuous repression of a tumor suppressor gene is required for a tumor to survive," said Dr Andrea Ventura, a member of the MIT team.
Cited story.
- Log in to post comments

You may have seen this article:
Development: p53 suppresses the self-renewal of adult neural stem cells
http://dev.biologists.org/cgi/content/full/133/2/363
"(N)eural progenitor cells retain the ability to proliferate and self-renew throughout adulthood and may be susceptible to convert into a malignant phenotype and may serve as an interesting model for the cancer stem cell hypothesis. The increased proliferation rate in the neural stem cell niche may elevate the risk of acquiring mutations, which could contribute with other previously described mechanisms ... to the increased predisposition to cancer in the absence of p53....
The functional analysis of other known oncogenes and tumor suppressors in stem cell maintenance, together with the present data, suggests that several molecular pathways in stem cell biology may converge on p53 for the control of stem cell self-renewal."
pretty cool stuff. I will have to check in on if/what the lab where I work is doing with p53.