By way of Effect Measure, I came across this Consumer Reports article about bacterial contamination of chicken. The short version: I'm not convinced that the bacterial contamination problem has become worse--keep in mind, I'm not saying that bacterial contamination is not a problem, only that the problem has remained constant in scope.
A good comparison to the Consumer Reports ('CR') study is the National Antimicrobial Resistance Monitoring System ('NARMS') report. For almost ten years, NARMS has been collecting bacterial isolates nationwide from store-bought meat products, including chicken, the subject of the CR report.
Right away, I was a little puzzled by a claim in the CR article:
In the largest national analysis of contamination and antiÂbiotic resistance in store-bought chicken ever published, we tested 525 fresh, whole broilers bought at supermarkets, mass merchandisers, gourmet shops, and Ânatural-food stores in 23 states last spring.
While the CR method covers more states than NARMS (23 versus ten), I think the NARMS sampling scheme is far superior. Every month, in each of ten states, a package of chicken breasts is purchased in ten different stores. This sampling scheme allows you to tease apart the effects of time and geography. "Spring" is a little less precise. So every year, NARMS is testing over 1,000 chickens (which is a little larger than 525). And NARMS does release a report every year, so I think CR would be aware of this.
But let's get to the guts of the article. I mentioned the sampling scheme in detail for a reason: there is tremendous variation in the contamination rates of Campylobacter and Salmonella in different parts of the country within the same year (this is from the NARMS 2004 report):
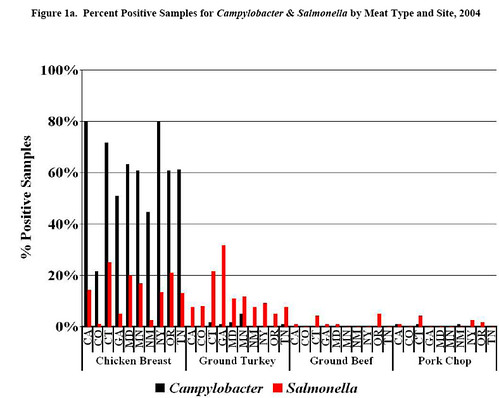
You'll notice, that in 2004, the frequency of Campylobacter contaminated chickens ranged from 20-80% and Salmonella contaminated chicken ranged from 3-18% depending on the state. In light of this variation within a single year, taking two time points and drawing any conclusions is dicey, especially since it's unclear if the geographic distribution of the samples from the two years in the CR report is the same. I'm not sure the dramatic increase in contamination claimed by CR is valid.
The CR report also discusses antibiotic resistance. Here's what CR reported:

click here for larger image
This is hardly shocking. In fact, the Campylobacter and Salmonella data look like... everything else I've ever seen from a U.S. agricultural setting. While some of the frequencies of resistance appear to be higher than the NARMS report, others are not (here are the Salmonella and Campylobacter data).
There's another thing that puzzles me about the data: the frequencies of resistance to ampicillin versus cefoxitin. Most resistance mechanisms (beta-lactamases) that confer resistance to cefoxitin also confer resistance to ampicillin; however, ampicillin resistance doesn't necessarily confer resistance to cefoxitin. Consequently, resistance to ampicillin is almost always more common in a given sample than resistance to cefoxitin (the same phenomenon also applies to ceftiofur). Yet, in the CR report, that pattern doesn't hold, which is puzzling (and potentially troubling).
For both the antibiotic resistance data and the contamination data, I'm just not convinced that what CR is seeing is anything more than variation due to differences in sampling (i.e., different geography and different chicken parts), as well as stochastic effects. Having said that, it should be noted that the contamination rates and level of antibiotic resistance in the U.S. are significantly higher than some other countries, such as Denmark. If the Danes can do better, why can't we?
A technical aside: I assume that the lab which conducted the testing used CLSI methods. It would have been nice if CR had put that information in a footnote. I'm also not certain what footnote 1 in the antibiotic resistance table means: "31 percent of samples were somewhat resistant: The antibiotic inhibited bacterial growth but did not stop it." I guess they're referring to intermediate resistance (when one tests for resistance, depending on the method, there are often concentrations at which the organism can't grow, but are well above the cutoff for clinical resistance.)
- Log in to post comments
