This year, several research groups have used bacterial proteins called channelrhodopsins to develop a technique with which light can be used to control the activity of nerve cells or the behaviour of small organisms.
For example, Ed Boyden's group at the MIT Media Lab used the method to activate or inhibit neurons on a millisecond-by-millsecond timescale, while Karl Deisseroth and his colleagues at Stanford have created an optical on/off switch that can control the movements of the nematode worm.
Devices employing such technologies could in theory be used in advanced neural prostheses for a variety of conditions, such as epilepsy and depression, and neurodegenerative disorders such as Alzheimer's and Parkinson's. However, there are several major challenge to achieving this.
First, the blue light used for photoactivation of the channelrhodopsin proteins is highly scattered by brain tissue, so, while the technique can be used in nematode worms , using it in larger animals is far more difficult. Secondly, it is not clear excatly how light could be targeted to the specific region of brain in which the channelrhodopsins are being expressed.
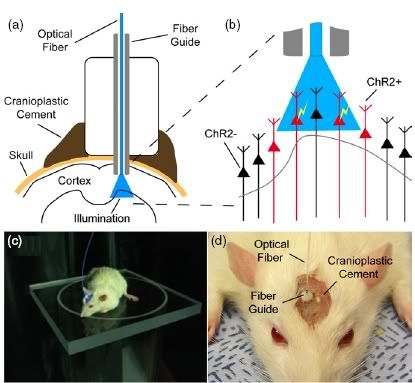
Deisseroth's research team has now taken this new technology one step further. In the Journal of Neuroengineering, they describe an optical neural interface which they used to control the whisker deflections in living, intact rats and mice.
Deisserdorf and his colleagues first delivered the channelrhodopsin (ChR2) gene into slices of rodent brain using the previously established method, in order to establish the extent to which light would be transmitted through the tissue.
They cloned the gene and inserted it into a viral vector, together with a genetic element called a CaMKII alpha promotor that drives the protein to be expressed specifically in neurons. Using a fiber optic guide which served as a cannula, the researchers then delivered the gene construct into motor neurons within the brain slices. Antibody staining showed that the ChR2 gene had indeed been delivered to the motor neurons, and electrophysiological recordings confirmed that the cells were activated in response to beams of blue light.
Once they were satisfied that this method could effectively deliver the ChR2 gene to the specified cells and that light could penetrate the brain slices sufficiently, the researchers then set out to develop a method by which the ChR2 gene could be delivered into the brains of living, behaving rodents. They targeted the vibrissal motor system, the part of the rodent brain that controls whisker movements. Thus, the level of optical control could be quantified by the deflection of the deflection.
The neural interface developed consisted of an optical fiber guide, fixed to the skull with a stereotactic rig (see above). The guide was first used as a cannula to deliver the ChR2 construct to into excitatory motor neurons in layer 5 of the vibrissal motor cortex. Then, an optical fiber was inserted into the cannula, so that the light beams were directed at exactly the same cells to which the ChR2 was delivered.
It was found that the interface could effectively control the output of the motor neurons in the vibrissal system. Small magnetic particles were attached to the animals' whiskers, so that their movements could be observed using a magnetic field sensor. Pulses of blue light delivered to the motor neurons via the optical interface caused the whiskers to deflect.
As in the earlier work, the ChR2 construct was delivered to a specific set of neurons. When expressed in the motor neurons, ChR2 made the cells sensitive to blue light, which could then be used to control the activity of the cells on a millisecond-by-millisecond timescale. Deisserdorf's team also used the optical interface to control the whisker movements of mice, whose brains are somewhat smaller than rats.
In the future, such techniques could be used to develop neural prostheses whose optics are either anchored to the inside of the skull or embedded with the brain tissue. Such optical devices would have a number of advantages over those used currently (e.g. the electrode arrays used for deep brain stimulation or the magnetic coils used for transcranial magnetic stimulation). Their size would be comparable to that of the electrode arrays currently being used.
For example, photoactivation can be used to excite specific cells, but electrode arrays cannot. Also, the electrode cables are susceptible to becoming encapsulated by glial cells within months of being implanted; optic fibers would be less so. And, the magnets used for transcranial magnetic stimulation can only deliver magnetic fields superficially; the current work shows that optical interfaces can be used to activate neurons within deep brain structures. Finaly, the long, light and flexible optical fibers would effectively keep heat-generating elements away from target tissues.
Reference:
Aravanis, A. M., et al. (2007). An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural. Eng. 4: S143- S156. [Full text]
- Log in to post comments
ghfg
Hello Sir,
My name is Craig J. Phillips. I am a traumatic brain injury survivor and a master�s level rehabilitation counselor. I sustained an open skull fracture with right frontal lobe damage and remained in a coma for 3 weeks at the age of 10 in August of 1967. I underwent brain and skull surgery after waking from the coma. Follow-up cognitive and psychosocial testing revealed that I would not be able to succeed beyond high school. In 1967 Neurological Rehabilitation was not available to me, so I had to teach myself how to walk, talk, read, write and speak in complete sentences. I completed high school on time and went on to obtain both my undergraduate and graduate degrees. For an in depth view of my process please read my post, http://secondchancetolive.wordpress.com/2007/02/18/my-journey-thus-far/
Through out my lifetime I developed strategies to overcome many obstacles and in so doing I have achieved far beyond all reasonable expectations. On February 6, 2007 at the encouragement of a friend I created Second Chance to Live. Second Chance to Live, which is located at http://secondchancetolive.wordpress.com presents topics in such a way to encourage, motivate and empower the reader to live life on life�s terms. I believe our circumstances are not meant to keep us down, but to build us up. As a traumatic brain injury survivor, I speak from my experience, strength and hope. As a professional, I provide information to encourage, motivate and empower both disabled and non-disabled individuals to not give up on their process.
Please encourage your readers to visit Second Chance to Live at http://secondchancetolive.wordpress.com
Thank you for your time and kindness.
Have a simply phenomenal day!
Craig J. Phillips MRC, BA
Second Chance to Live
Our circumstances are not meant to keep us down, but to build us up!
This sounds pretty exciting! I was just wondering about one thing though. How long before the injected ChR2 construct breaks down? Or does it keep expressing stably for good?