This series of posts is intended to explain the tools and tricks used to create and manipulate samples of ultra-cold atoms; thus, it's appropriate to start with how we get those atoms in the first place. This will be a very quick background on the basic force used to make atoms cold, and then the technology of atom sources for a variety of experiments.
Okay, so you've got two things in the post title. Which are we going to talk about first? Well, the study of cold atoms really begins with the observation that light can be used to push atoms around. There are actually two ways to do that, but one of them is more directly applicable to cooling, so let's start there.
So, this would be "light scattering forces," then? Okay, what does that mean? It sounds like you're using a force to shoot light in all different directions. That's almost exactly backwards-- these are forces that arise from the scattering of light. Specifically, the absorption and emission of photons: when atoms absorb and emit light, they experience forces that push them around, and cold-atom physicists use those forces to make ultra-cold samples.
Okay, that makes no sense at all. When I absorb light from the Sun, I don't feel a force, or get cold. In fact, I warm up. So how does absorbing light make stuff cool down? It's true that in general, solid objects absorbing light don't experience a significant force, or cool down. It's only in very specific, carefully arranged circumstances that you can exploit these forces to do actual cooling.
The trick relies on an idea that celebrated its 100th "birthday" this year, namely Niels Bohr's first quantum model of the hydrogen atom, which was based on the idea that atoms move between states by absorbing light of particular frequencies. This is most easily understood as a quantum process, though some curmudgeons insist on pointing out that the process can be understood semi-classically, so we'll talk about this in terms of photons and the absorption thereof.
When you think about light in terms of photons, each photon carries an amount of energy determined by its frequency (the color of the light, loosely speaking). This is the key to the Bohr model, and Einstein's model of the photoelectric effect, and the whole origin of quantum mechanics. What's less often pointed out, though, is that these photons also carry momentum.
Wait, I thought photons didn't have mass? Isn't momentum mass times velocity? In classical physics, we usually think of momentum as mass times velocity. In a deeper sense, though, momentum is a more general property, and anything with energy will also have momentum. In fact, you can easily show from Einstein's relativity that a massless particle will have momentum equal to its energy divided by the speed of light.
The key to light scattering forces is that when an atom absorbs the energy of a photon of light, which it uses to boost an electron to a higher energy state, it also absorbs the momentum of that photon. That momentum gives the atom a "kick" in the direction the photon was moving.
Wait, why haven't I heard this before? This wasn't part of the Bohr model when I learned it in grade school. It's not generally mentioned because the "kick" is really, really small. Rubidium is one of the most commonly used atoms in cold-atom experiments, and the change in the velocity of a rubidium atom absorbing a single photon is about 6 mm/s. That's way to slow to see in a single experiment with a room-temperature sample, so most treatments of absorption and emission ignore the effect.
(If you talk about really high-energy photons, the momentum does become important, and is critical for understanding the Compton effect. But you probably didn't learn about that in grade school, and it's not often highlighted in discussions of Compton scattering.)
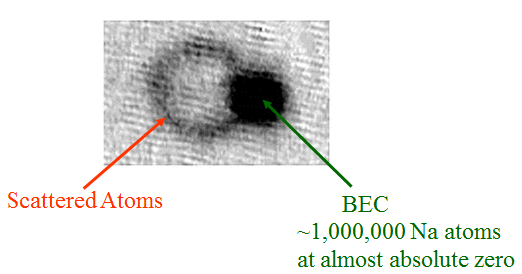
If this force is real, prove it. Okay, here's a picture showing the effect, taken from a paper by the BEC group at NIST:
This shows an image of a Bose-Einstein Condensate (about which more in a later post) after being hit by a short laser pulse coming in from the right. A bunch of the atoms in the condensate absorbed one photon each, giving them a "kick" to the left; some time later, when they snapped this picture, those atoms had moved leftward. The BEC itself is so cold that the tiny velocity change was enough to separate those atoms from the original cloud, making the ring on the left side.
Why is it a ring, though? Why not just a second blob? Well, because the atoms that absorbed a photon move one electron up to an excited state, but it doesn't stay there. A short time later-- around 16ns, give or take a bit-- the electron drops back down, emitting a second photon in a random direction. That second photon also gives the atom a "kick," in the direction opposite its motion, and as a result what would have been a second dark blob gets blown up into a ring.
Okay, that's pretty cool. So you can use light to exert tiny forces on atoms that are already cold? Well, you can use a single photon to exert a tiny force on a single atom. But here's the thing: photons are really cheap. A red laser pointer that you can get for next to nothing at your local office supply store will put out 1,000,000,000,000,000 photons per second. Given a moderately powerful laser, you can add up the forces from all those atoms to get a pretty substantial change in the velocity of an atom.
Right, so if you have an atom sitting still, you can give it a big push. But how does that make anything cold? It's not just atoms sitting still that get the push: you can also push atoms that are moving, and make them slow down. Since temperature is a measure of the average kinetic energy of the atoms in your sample, that amounts to cooling them down. And if you're clever, you can arrange to only slow the atoms down, not heat them up.
By, what, making sure to only zap the right atoms? In a sense, though not the Maxwell's demon kind of sense you're thinking of. You can get this automatically using the Doppler effect.
That's the thing where an ambulance siren coming toward you sounds higher in pitch, but an ambulance siren going away sounds lower in pitch? Exactly. The same thing works for light. Atoms moving toward the laser see the frequency of the light shifted up, and atoms moving away from the light see it shifted down.
And here's the thing: those atoms only want to absorb a very specific frequency of light. So if they're moving too fast in one direction or another, they won't absorb light that's tuned to the frequency that stationary atoms like to absorb. They will, however, absorb light tuned to a slightly different frequency, depending on the direction of their motion.
So, what you do, is you tune your laser to a frequency that's a little bit too low for a stationary atom to absorb it. In that case, atoms that are moving toward the laser will see the frequency shifted up, into the range that they want to absorb. Whereupon they absorb photons, and get "kicks" in the direction of the laser. But since that's the opposite direction to their motion, the resulting force slows them down.
Not only that, this force won't do anything other than slow down moving atoms. Atoms that are already at rest won't absorb the laser, and atoms moving in the same direction as the laser see the frequency shifted down, even farther from the frequency they want to absorb. So you get a cooling force more or less automatically.
And that's how you make cold atoms? You start with a bunch of atoms going in the same direction, and blast them with a laser at a too-low frequency? That's one way to start the process, at least. The too-low frequency part is referred to as "red detuning," by the way, because red light has a lower frequency than blue light, and you've deliberately de-tuned the laser away from the natural frequency of the laser.
So, how do you get a bunch of atoms moving in the same direction for you to stop this way? Well, that's the "atomic beam" part of the post title. And it's actually pretty easy to do-- you just take a box with a gas of the appropriate atoms in it, and make a hole in the side. Atoms come spraying out of the box through the hole, headed more or less in a single direction. If you want to be more selective, you put a second wall in with a second small hole in it, and only atoms that pass straight through both holes will make it past (provided, of course, that you do this inside a vacuum chamber, and pump away all the atoms that don't make it through both holes on the first attempt), giving you a "beam" of atoms all headed in the same direction. Then you shine your laser in the opposite direction, and slow the beam down.
There's a problem with this, though. Yes, what's that?
Well, you're relying on the Doppler effect to guarantee that you only slow atoms down, but the speed of the atoms is changing. Doesn't that screw things up? Excellent catch. You're absolutely right, the simplest version of this is self-limiting: you blast your laser into a beam of atoms, and they slow down a bit, but once they do, they stop absorbing light and thus stop slowing down.
So how do you fix that? Well, there are two things you can do. One is to change the laser: as the atoms slow down, the exact right frequency to get them to continue to absorb light and cool down changes. So, if you change the frequency of the laser, you can follow the motion of a particular bunch of atoms, and slow them down much more than with a fixed frequency. This is called "chirp cooling" of an atomic beam, because the frequency change is analogous to what birds do when they sing.
That only works for a particular bunch of atoms, though. What happens to atoms that aren't at just the right speed for a particular point in the chirp? Well, they don't slow down. This is inherently a pulsed method, giving you little bunches of slow atoms at the end of every chirp. Which is why it's not terribly popular these days.
So what's the other option? Well, there are only two things involved in this process: the laser and the atoms. If changing the laser doesn't work, the only other thing to do is change the atoms. Which you can do by applying a small magnetic field that shifts the energy states, and changes the frequency the atoms want to absorb. You can use this to compensate for the changing Doppler shift.
So instead of changing the frequency of the laser, you change the magnetic field that you apply? Doesn't that get kind of annoying? Actualy, yu can use a fixed magnetic field, because the atoms are moving while they slow down. So, when they've just come out of the source, they want one magnetic field to match the laser, but when they've slowed down enough to require a new field, they're in a different position. So you can use a single laser frequency, combined with a magnetic field that varies in space-- a tapered electromagnet will do the trick very nicely.
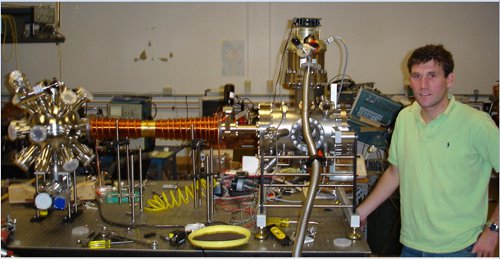
This technique is called "Zeeman slowing," because the change in energy levels due to a magnetic field is the "Zeeman effect" (pronounced ZAY-mahn, because he was Dutch). It's amazingly robust, and inventing it got my thesis advisor a share of a Nobel Prize. you can see a picture of a Zeeman slowing magneti in the "featured image" up top, or right here if you don't like scrolling:
The shiny copper wire to the left of Ryan in that picture is the Zeeman slowing magnet he built as part of his undergrad thesis. You can see that the thickness of the coils varies from point to point, which produces a magnetic field that changes as you move along the pipe. For the right choice of currents, it will exactly compensate the changing Doppler shift, and keep the atoms slowing down all the way down the pipe.
It's important to note here that the slowing force is really coming from the light. The magnetic fields used are pretty small, enough to shift the energy levels, but not big enough to produce a significant magnetic force. If you use whopping huge fields, you can slow atoms just with magnets, but for that you want Mark Raizen's group at Texas.
Okay, so that gets you a bunch of atoms that are moving in the same direction at a low speed. What's this good for? Well, you can use slow beams for a bunch of things-- low-energy collisions between crossed beams, for example. But the real usefulness of these is as a starting point for other methods that will bring those slow-moving atoms to a stop in a particular place. But--
Let me guess: that's the next post. Yep. We are, after all, 2200 words into this. I promise the next one will be shorter, though, now that we've got this background out of the way...
- Log in to post comments

In the 9th paragraph, did you mean 6 mm/s or is it really 6 nm/s?
You've got a green shirt at the top, and Ryan's wearing one too. Is that the lab uniform?