Let me give you a little back story. As many of you know, I'm new to scienceblogs, and one of the first things we get to do is join a message board full of all the bloggers (sciblings) here. Well, suffice it to say, a contentious discussion took place between me and another scibling, which resulted in my being called a total n00b in front of the entire world.
So, what better way to make peace than by having me -- an astrophysicist -- answer a question about physiology?! Let's take a look at what I've got, since I'm a frequent solicitor of questions:
Why is my pee yellow in the morning, but clear in the evening?
The human body is a favorite topic of mine, and has been ever since I answered the question of whether a human has more cells or a galaxy has more stars? Well, let's take a look at what's in your pee:
Sure, a whole lot of ions and chemicals, salts, proteins, enzymes, hormones, and other metabolites are found in urine. But what makes it yellow? The distinctive yellow color comes from urobilin, which is a chemical formed, eventually, from the breakdown of hemoglobin. You can even (kind of) see how it happens; start with a heme group:
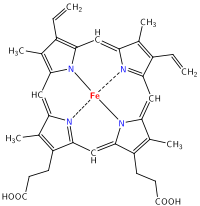
and then break it down and remove the iron! Take a look at how alike these molecules are:
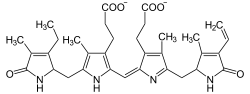
So when your urine contains a lot of urobilins, it's quite yellow, and when it's low on urobilins, it's closer to clear. So that's the easy answer, but we can dig a little deeper. First off, what are we doing destroying all of this hemoglobin? Don't you need it to live?
Of course you do, but your circulatory system is constantly ordering replacement parts. Your body is constantly making new red blood cells, replacing the old ones every 4 months or so.
But there are about 30 trillion red blood cells in the human body, meaning you are both destroying (and making) new red blood cells at a rate of around 2.7 million cells per second. What's more, is that every red blood cell has about 270 million hemoglobin molecules in it, with each one capable of carrying four oxygen molecules (and having four heme groups).
All told, assuming you sleep for 8 hours at night, your body has destroyed 78 billion red blood cells, now has to get rid of 8.4 x 10^19 heme molecules, and has ingested no water over that time. Now, most of these molecules wind up in your stool instead of your urine, but they've been able to determine that typical human urine contains around 8 mg of urobilin, which equates to about 10% of the broken-down heme molecules winding up in there.
But over the day, you're drinking water, and so you're diluting the amount of urobilin in your urine by a significant amount, allowing you to pee clear by the afternoon under normal circumstances. Someone like me -- who drinks about 4 liters of water a day -- gets their evening urine down to about one-sixth of the urobilin density found in their morning pee. Put simply, the determining factor for healthy people is hydration.
And so my friendly, horizon-expanding challenge back to you, anonymous scibling, is for you to solicit and answer a physical science question of your choosing! If you'd like a little help, I can point you to the most recent Carnival of Space (#97) for inspiration!

You've got a whole Universe out there to choose from...


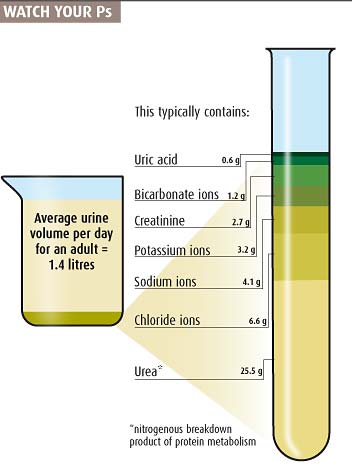
I like the urine composition chart: nicely and funnily illustrated; even if it does give one the image of lab coats comparing 1L graduated cylinders full of urine.
Now there is one detailed CV.
I however prefer startwithabang.com than scienceblogs.com over here. So you're like restricted to post non-science articles here?
Hey there Sophos! Good to see you on the new site. While I like my old background better, the community here is a little more fun than just whatever I could create on my own.
I can still post whatever non-science articles I want, I just need to put them into the appropriate categories. But, it hasn't been very long -- I'm sure there are plenty of non-science articles that are going to come out of me.
Damn.
It shows that I never paid any attention in anything bio.
I had no idea it was that many.
So if I understand this right this guy is upset with you because you didn't list his blog in your list of favorite science blogs? To me putting up a post bashing someone simply because they didn't publicly say how wonderful you are sound a bit juvenile. I think this guy has way to big an ego.
I never thought about why urine was yellow before. I find it interesting that the hemoglobin ligand without iron chelated to it gives it a yellow color, as the pyrolle groups themselves act as chromophores.
Voldemort, what kind of reverse sexism is that, assuming just because she has such an ego that Dr. Isis must be a man?
(I'm just kidding, but she really is female.)
And yes, Lt., it's totally weird how just a small chemical change will change the color completely of a molecule!
It's not that odd, as the vast majority of colour in compounds is caused by the existence of transition metals, and their chemistries (their ionisation state and the chemistry of their ligands). So it's not that surprising that loss of the metal ion would cause a dramatic change in the colour of the molecule.
After all binding of oxygen to your hemoglobin changes it from a strong blue (deoxygenated blood, in your veins) to a strong red colour (oxygenated, in your arteries)!
That's because metals themselves, when complexed by ligands, exhibit colors due to their d-orbitals having electrons excited by incoming photons, and then photons ejected, as well as by electrons jumping from metal orbitals to ligand orbitals and vice-versa.
But that's not the only type of chromophore; the other type are ones with conjugated pi systems, such as the urobilin. Virtually any compound with a conjugated pi system is a chromophore (even benzene acts as one to UV light). What I never thought about was that without the Fe in it, the urobilin it gets converted to by breaking the bonds that keep it as a prophyrin ring can get oxygenated itself (such as when expelled from the body) and acts as a chromophore then due to its tetrapyrrole system.
That's not true. Deoxygenated blood is still red, just a darker red. Blood never turns blue; veins are blue because of several different reasons (partly because of the bright red oxygenated blood not helping color them), but it's not because deoxygenated blood is blue.
If you've ever given blood, you would've seen proof of this, though it may not have been apparent. The blood they took was deoxygenated, as it came from a vein, but it was still a very dark red color.
Besides, blood is never fully deoxygenated unless you're suffering from acute carbon monoxide (which actually turns blood bright red, because it's still oxygenated in a way, it's just the CO holds the oxygen into the heme group and won't let go).
As an addendum to my last post, a better example of how small changes can result in very different colors, I offer the example of biliverdin, a different potential by-product from urobilin that has a green color to it rather than yellow;
http://upload.wikimedia.org/wikipedia/commons/3/3e/Biliverdin3.svg
If you compare that to the urobilin diagram in the original post you can see the only differences are that the carboxylate groups are protonated, and one of the methyl groups is replaced by a CH2 group (which I can't remember the correct name for. Olefin group, maybe?), which adds another pi system to the conjugated system of the molecule and shifts the color it exhibits a few hundred nanometers in wavelength.
Whoops, small mistake in my last post; it'd shift the color about 60 nanometers. It'd be a few hundred angstroms the color got shifted.
LtStorm:
You are very correct, I had completely forgotten the red->dark red change on deoxygenation of haemoglobin. I was aware of this, in respect to the ionisation state of Fe in the heme group, yet for some reason still had it stuck in my head about deoxygenated blood being blueish.
I think it was from a story about how veins appear blue, yet when you cut one the released blood is red, which I was told in GCSE (15 year old) biology, and that this first led people to think that it was something in the air that changed its colour, leading to some of the first ideas about blood moving oxygen around the body (although they didn't know what in the air it was that caused the change).
I remember an experiment where the experimenter cut a vein in a bell jar that had had a candle burnt in it until all the oxygen was used up and the candle burned out, and it was noted that the blood was a different colour. This obviously led to the conclusion that the candle was using the same gas in burning that blood was carrying round the body.
I should have also mentioned conjugated pi systems as the other type of chromophore. I always think that it is so cool that you can look at something with colour and know that one of the two are at play.
Do you know of any examples where both are occurring? Where light interacts with the metal center AND the conjugated pi system of its ligands?
Well, technically both are happening in hemoglobin itself. I believe having both in play means you see the predominantly absorbed color (well, the complement of the predominantly absorbed color; hemoglobin absorbs green light, which makes it look red, if you consult a color wheel), but with the secondary color adding, and shifting the exact color of the complex slightly.
I could be wrong on that, but it seems right.
A more interesting case is a molecule I've been working on; a conjugated pi-system chromophore attached to a ligand (called a siderophore) that chelates a Lanthanide.
Lanthanides can't fluoresce on their own due to the Forbidden Transitions, as laid out in quantum mechanics. One way to get around this is to chelate them to the aforementioned pi-system, the chromophore acting as an "antenna" and using charge transfer through the conjugated pi-system to cause the Lanthanide to skip those forbidden transitions and fluoresce. Very interesting stuff.
Wonderful post!
I really liked this post..!!! I enjoyed it, cause now I know why urine is yellowish, and so I know I'm not dehydrated =]
cool, and thanks for posting this :)
I like this web..thanks so much.
i´m a mexican student.
Here's a puzzler, to which I found no direct result in a Google search... why, when I pee at night without flushing, does the yellow pee in the bowl turn clear by morning?
My toilet sometimes runs, and going to bed, I don't want to stay awake to monitor and fix it, so sometimes I pee (normal yellow) and don't flush. Then, in the morning, the bowl is clear. But not running, and it didn't run the night before, so it's not because of any steady flow of clear water from the tank.
What makes normal yellow pee turn clear over time?
Yester morning to noon it was very dark urine in colourvand know I woke up from bed went for a pee and noticed my urine was like yellow completely yellow and I could just believe it was a urine.
Wow! So much I didn't know. It is sort of disgusting, I guess, but you have to learn! This is amazing!
sheesh, don't encourage him!
p
Weird necromancing of the comments aside, this post is certainly an interesting historical snapshot.
It seems to happen a bit here. Nothing other than "Wow, so cool!", for no reason whatsoever, necroing a thread.
They will never be seen again.
I don't think it's necroing, since I at least got here from googling to find out why my morning pee is darker yellow and evening pee clearer, which I assumed was natural yet here we are because of curiousity, and it's well explained.
Don't care for that beaker graphic though. The top of it should start at the 100% containment/parts level of it; it shouldn't have an inexplicable light blue area between the top and first ingredient in pee. What is in my pee in that blue area? Is it Klingons celebrating their transition to Sto'Vo'Kor? Are Klingons in my pee? The graphic leaves that blue section in extreme doubt. My pee might also make up 30 or so % of nanobots whose sole design is to reenact Nic Cage movies. With that graphic, who can tell what is true about that mysterious, completely unknown blue section of pee?
If you have a beaker, vial, or measuring cup as a graphic, for god's sake fill it to the top. Baffling that this didn't, and really made me question my pee. I regret even coming here, so many sleepless nights ahead.