The Earth is one of those extremely rare, special places in the Universe where water can exist, stably, as a liquid. So much of it exists here on Earth, that if you were to add up all the oceans on Earth together, it would weigh more than 10^18 tonnes, more massive than the biggest asteroid ever, and about as massive as Pluto's giant moon, Charon.
But water only has a very small window in which it can be a liquid. For instance, if you took some warm water up to a very high elevation, it would start to boil, and become a gas! The higher up you took it, the lower and lower your boiling point would be.
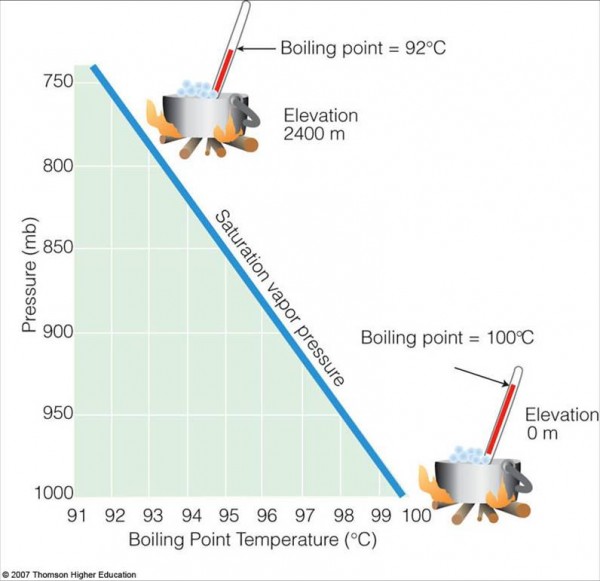
Why? Because higher altitudes on Earth mean lower pressure. If there's not enough force pressing the water into a liquid phase, then there's no force binding the water molecules together. If you simply allow them to diffuse away, they will. And that's the definition of a gas, which is what you'll wind up with.
On the other hand, water has no business being a liquid at low temperatures, either. You can see -- from this diagram below -- that if you start with liquid water, you can turn it into a gas by lowering the pressure, but you can also turn it into a solid by lowering the temperature.

So my question is this:
If you took a glass of water into outer space, would the water freeze or would the water boil?
This is a question that seems awfully tough, because in addition to knowing about water:

We also need to know about outer space. Space is a lot of things: cold, dark, and empty come to mind right away. And they come to mind, pretty much, as soon as you leave the Earth.

Well, the temperature of space is, at its coldest, just the temperature of the leftover glow from the Big Bang. This radiation, known as the Cosmic Microwave Background, bathes the entire Universe in a temperature of only 2.7 Kelvin. That's less than 3 degrees above absolute zero, or -455 degrees Fahrenheit! But there's also -- literally -- no pressure in space. So, what happens? Who wins? Does the water freeze or boil?
Oddly enough, the answer is first one, and then the other! It turns out that having a pressure vacuum will cause the water to boil almost instantly. In other words, the effect of boiling is much, much faster than the effect of freezing.
But the story doesn't end there. Once the water has boiled, we now have some isolated water molecules in a gaseous state, but a very, very cold environment! These tiny water vapor droplets now immediately freeze (or, technically, desublimate), and become ice crystals.

We've observed this before. According to astronaut observations, where they've observed their urine get expelled from the ship:
When the astronauts take a leak while on a mission and expel the result into space, it boils violently. The vapor then passes immediately into the solid state (a process known as desublimation), and you end up with a cloud of very fine crystals of frozen urine.
Sounds like it would be a fantastic thing to watch, doesn't it? Well, we've done almost the same thing on Earth. What happens if you take boiling water and, on a very, very cold day, throw it up into the air?
- Log in to post comments


I have tried this at home! But then, I do live in Canada...
That was brilliant! :)
For a practical application of Fig 2, move to Los Alamos, NM. At 92 C, you boil spaghetti al dente in 20 min, and your potatoes take an hour plus.
We used to do this as kids growing up in MN. Science, sometimes, is so much like magic :)
Why not get a pressure cooker, then?
That snowflake looks disturbingly familiar. Did you get permission from the photographer? If it's who I think it is, I'm sure he'll be happy to let you use the photo (and you can put a link to his web page where many high quality photos can be found).
@Mu: A good quality pressure cooker with a pressure adjustment works very well - if you can find it. You can just crank down the pressure setting so that water boils around the usual sea level boiling point. I'd take a normal pressure cooker and modify it, but I'm not saying anything because I wouldn't want to be sued if something goes wrong. :P
MadScientist,
Nope, don't know the photographer; just took it from google images. If you know the guy, send me his site info (and/or a link to his photo) and I'll link to it in the article.
As for the snow/boiling water trick, it seems that about -40 degrees is where you want to be for this stunt to work. I should've tried it the one winter I spent in Madison, WI...
http://www.straightdope.com/columns/read/118/would-a-glass-of-water-in-…
Check out this old straight dope, which happened to recently be reposted to the front page of straightdope.com
As for the pressure cooker suggestion, it's the perfect way to produce mashed spaghetti and al dente potatoes, due to problems of getting the timing just right. Also, I found starches in certain kind of pressure cookers quite dangerous, since the foam gets into the valve mechanism and makes them sticky.
For a funny anecdote, when I first moved their I grabbed a pack of cake mix and looked at the "high altitude" instructions, which were for 3000 - 6000 ft. I was baffled what to do at 7500.
Ethan,
You write the coolest blog. I learn something new almost every time I read it. I've always wondered what happened first in space - freezing or boiling.
I should have guessed, because every material has a thermal capacity, i.e. an ability to retain heat. So it stands to reason that it would respond first to the instantaneous drop in pressure, and then as it gives up its heat more slowly, drop in temperature to the freezing point. Makes sense, but I never stopped to think about it. I thought the "Urean Nebula" just went straight to ice, and I wondered why it didn't boil.
Answer: It did!
Forgive my ignorance - where does the heat in the liquid go that quickly?
The heat gets lost during evaporation and consequent expansion of the resulting gas into the vacuum.
thats not a snow flake ...its a pee flake....
hei..would you like to teach me how to make 2nd diagram (liquid,gas and solid)??
reply me pls.thanks
Hi,
can u say me where does the heat in the liquid go quicly ?
Thanks i haven' understand
Enjoyed the post. I was curious as to the final droplet size distribution. I assume that once they are fully cooled, sublimation drops to a very very low rate due to the extremely cold temperatures. My interest: contaminant movement in space and possible incursion into space shuttle bay.
that help me alot but Seriously it helps cuse my teacher ask me so i finely have the answer...tnx¡
⢠This article makes me wonder as to how water first came into existence, while helping to further educate about water in the our galaxy and universe.
On the same subject, we have the following quotes from the award-winning philosophical, spiritual, science exploration book published in 2001 called, "The Holy Order of Water, Healing Earth's Waters and Ourselves":
"Cosmic clouds containing water were recently discovered rotating around black holes that are suspected to be near the center of our universe. These cosmic clouds of water indicate the creation and distribution of water throughout our universe at an early age.
⢠One water distribution method we find throughout universe occurs through comets. Water is one of the major constituents of comets - since they are made up of about 90% water in the form of ice mixed with dust. âWhen Halley's Comet last visited our solar system in 1985, scientists reported that 'the comet's dominant constituent is water ice, and that much of the tenuous 360,000-mile-wide cloud surrounding it consisted of water vapor.' At one point, it was estimated that the comet was losing up to 70 tons of water a minute!â
I really mean what would happen if water is heated in space. If it turns into gas how?, If stays in same position how,? water must change into gas on heating but to it goes with moving up and down. But in space this process does not take place as there is no gravity.
Water turns into crystals in space. crystals are snow. Snow sticks to snow. I'm wondering if this could be a clue to how planets form in the solar system. There seems to be plenty of water in space. Especially on planets and moons. Could the mystery of how planets form be solved by the fact that snow is sticky? Crystals smashing into one another forming snowballs. The snow balls grow and grow till they have enough gravity to attract dust. Anyway I've heard it said that people don't seem to know how planets are able to form as gravity is not enough to cause dust to clump together.
i am telling that in under the earth there is no water, because h2-hydrogen is only available and wheno-oxygen enter then its changed into water=h2o.
yolo you only live once
ohh .very tragic to know bout water
I can understand water boiling in space but I dont understand the freezing part. How does the liquid pass its energy in a vacuum when there are no adjoining particles ?
Ok I will also speculate that what the astronauts observed was not frozen Urine, it was a liquid crystalline formation that appeared to be solid.
What effect if any would viscosity have on freezing/atmospheric pressure of water-based liquids? In orbit if an astronaut opened a squeeze bottle of honey, would the honey boil out then crystallize in a fine cloud?
If you had a squeeze bottle of salt water and exposed it to space, would the minerals from the salt water be left at the bottom of the bottle? Like seeing sea salt and stuff?
If this is what happens to water in space, how are comets explained then? Aren't they mostly ice? How is this possible?
Jim Hinkey,
Did you read the article? Water in space first boils, then freezes. Thus, it ends up as ice, just like the ice that makes up the comets.
Russell Bamberger,
Freezing (technically desublimation, but I'll stick to freezing here) requires that there be a heat transfer from the water vapor that is doing the freezing to the surroundings. No heat transfer, no freezing (otherwise the second law of thermodynamics is violated). This can be accomplished by radiation, namely by emission of infrared photons. This certainly can occur without any surrounding medium.
I have two say amazing:):):):):):):):):):):):):):);););)@ya
Do you have any other science project for my son,he's a teenager so it's hared getting a good science project.
No I don't have any more science projects
If people are worried about water levels rising because of global warming why don't we bring water to outer space so our land won't be compleatly covered.. Maybe I sound stupid saying it. Yes it will turn to gas then freeze but if scientest find a container that can hold the molecules in place that might work. Just a thought
My question- hypothetically if there wasn't any external conditions like pressure temperature and gravity affecting waterm then will a droplets of water remain static in vacuum? I personally think it wouldn't stay static because of its internal energy and would be floating around this leads to a point that no matter in the universe stays static and is trying to spend its energy in a way ..if im wrong pls do correct me.."prmahesh007@gmail.com
Thanks for an elaborate explanation.
Hello, does anyone know, in relation to the graph on here, what the sp, mp, and nbp mean? I was thinking melting point and such, but then nbp doesn't make sense for me, although the sp is an arguable point. Hence my confusion.
It was very useful and interesting.
So my question is as a refrigeration engineer, if we have standing water in a closed circuit and place this circuit under a vacuum of say 500 microns, will the water just boil away rapidly or will it eventually turn to ice? If we based my question on the pipe work and surrounding air of around 5c?
As a refrigeration engineer, you should either be able to answer that question yourself (in the case the qualification is relevant enough to merit display) or it wasn't really germane to your query.
First, what do you mean by "under a vacuum of 500 microns"? mmHg? Secondly, absent knowing that, you've made it stable at 5C, so looking at the phase diagram of H2O answers that.
it has broaden my mind,thinge looks simlpe, but chemistry of space is yet not clear to me,instead of water, if there is heavy water H2O12,THEN SCENORIO will be different
I think it will boil in space
I think it will turn in to gas
I think it will turn in to gas
And I think it will write a stern letter to the Daily Mail about how the poor are scroungers.
Guys, it's not a vote. "Think" means nothing unless you put some actual thought behind it.
i think the same above wow so cool
`
So what happens if you fill a water bottle, seal it and expose it to space? For the water in the bottle to cool down, it needs to lose the heat through the conduction through bottle walls to outside environment, where the heat dissipates through convection.
Since there are no molecules in space, how does the heat in water get dissipated?
The bottle will lose heat by radiation.
@Khan #40
'(H2O)10 and (H2O)12 are used to investigate the growth of ice on metal surfaces with hexagonal symmetry.'
Refer http://www.ncbi.nlm.nih.gov/pubmed/17154569
if we create vaccum in a container on earth and then pour a water in that container will water start boiling and form vapour???
Thank You!
Aiman,
A qualified yes. Qualified in that it depends on temperature. There is a property of water (and any other material) known as vapor pressure. The vapor pressure is the equilibrium pressure that will result from placing the material in a closed, totally evacuated container. The vapor pressure depends on temperature - higher vapor pressure for higher temperatures.
The way this relates to your question is this: if the pressure of the vacuum into which you place your water is lower than the vapor pressure of water at the temperature in the sample, the water will boil. You can boil water at room temperature if you reduce the ambient pressure to below the vapor pressure of water. If your sample is cold enough, and your vacuum is imperfect enough, the water will not boil. If the vacuum is good enough and the water warm enough, then yes, it will boil.
The Laws of Nature so fascinating. Wish, I did try it but I am in LA CA always hurting for rain :(
So could you use that boiling point to produce energy for faster universe exploration?
Please don't say Big Bang as a fact.
Soooo.. would am organism. Eg human, eplode in to crystal vapor?
They have skin and so forth that contain the fluid, whereas water droplets only have surface tension.
"Please don’t say Big Bang as a fact."
OK, I can still say it's true, then. Please don't say I'm wrong.
Hi. I would like to know if it would be the same scenario with large amounts of water. I am imagining a huge space ship during an interplanetary travel. Assume that is moving in the vacuum of the space with one of his chambers totally filled with 30.000 galloons of fresh water. Let me also assume that suddenly some astronauts decide to do scuba diving in the middle of the mass of water. Then a terrible accident occurs: the pressurized chamber opens and all is ejected to the vacuum. My question is if it might be possible that in the surface of the ejected mass of water, after boiling, a frozen crust can be formed permitting to maintain the divers alive in the liquid core, at least while they can hold their breaths.
"OK, I can still say it’s true, then. Please don’t say I’m wrong."
OK, I can still say it's all in our binary imagination. Please don't say I'm wrong.
OK, you're full of crap.
There, I didn't say you're wrong!
Oh, and well done being too ignorant to know what I did there. Just what we expect from a creotard like yourself, teabaggie.
"Oh, and well done being too ignorant to know what I did there. Just what we expect from a creotard like yourself, teabaggie."
Well must be because I don't sit on my fat ass all day living vicariously through a pseudo persona via science blog created by a GREAT AMERICAN that as usual you have to ride the coat tails of others rather than to create your own work.
L....O....O....S....E.....R.........
"Well must be because I don’t sit on my fat ass all day"
So your "employer" as well as locking you in a bunker doesn't give you a chair, hmm?
"living vicariously through a pseudo persona"
Irony. See above.
"that as usual you have to ride the coat tails of others rather than to create your own work."
Irony 2. Irony harder.
"L….O….O….S….E…..R………"
It's *always* projection with you retards.
Terrified to find the world isn't as you thought it was, you cling with bitter hate to your internal monologue and think it's the Voice of God(tm).
“Well must be because I don’t sit on my fat ass all day”
No, it; because you're a moron.
Sorry to bear the tidings of bad news to you. On the upside, you're a good fit for republican voter. Much easier to lead you around by the nose if you don't bother to think, and couldn't manage it in the first place.
L O O S E R ??
Sure you didn't mean LOSER?
Big difference of meaning for the 2 words, RM.
"Much easier to lead you around by the nose if you don’t bother to think, and couldn’t manage it in the first place."
haha What a canard.. Heck anyone who would vote for a Clinton is a brain dead idiot blind follower.
"Sure you didn’t mean LOSER?"
Nope and if you want to get technical it's actually
LA'WHO-Z'A'HER :)
https://www.youtube.com/watch?v=0Kz7YUdy-Cg
"haha What a canard..
What duck?
"Heck anyone who would vote for a Clinton is a brain dead idiot blind follower."
Irony 3: Irony with a vengeance.
"if you want to get technical "
No, we want to get ACCURATE. Not faked stuff. Like your religion.
"No, we want to get ACCURATE. Not faked stuff. Like your religion."
Accuracy? OH, you mean the truth. Well you can't handle the truth, you prefer lies and deception.
And since you brought up, "my" religion when in fact it's all ours.
I will help you out with a verse or two that proves you can't handle it ( I'll even use the King James Version).
Romans 1:24-25 "24 Wherefore God also gave them up to uncleanness through the lusts of their own hearts, to dishonour their own bodies between themselves: 25 Who changed the truth of God into a lie, and worshipped and served the creature more than the Creator, who is blessed for ever. Amen.."
There's your truth. Merry Christmas and Happy Hanukkah Wowzer the Pagen
Jim Carey isn't a good representation for anything, really. But, what the heck; he gets paid more than I. So much for your pagenisms - sick; just sick (as in bad). May the bird of paradise fly up your nose, RM.
"So much for your pagenisms "
Urr Umm, Sure you didn’t mean paganism? :)
"May the bird of paradise fly up your nose, RM."
PJ Really? Your slumming now with the likes of Wowzer?
She is someone you uphold as a model secrete poster on a Science Blog?
She is a whore, you would be wise to run from her least her talons of ilk grab holt of you.
”Well you can’t handle the truth, you prefer lies and deception."
It;s ALWAYS projection with you creotards.
"And since you brought up, “my” religion when in fact it’s all ours."
No it isn't. Your religion has just you in it. Which words are "canon" what they mean, which ones you think you should obey, what precisely the trinity is, who else is actually in your faith, all conspire to make your religion a honed myhtos that has only one adherent: you.
Is Wiccan your religion? Because they say you can have it if you want. How about the polytheistic beliefs of the aboriginal australian? That's your religion too, if you want it.
If none of those over 6000 other religions aren't your religion, then you must have to accept it for it to be yours, in which case, yours isn't my religion.
And from the KJV:
https://www.youtube.com/watch?v=zEnWw_lH4tQ
Eat the flesh of the women???? Cut off their hands?!?!? No way that's worth listening to, load of cock that it is.
"Urr Umm, Sure you didn’t mean paganism? :)"
Urr Umm, don't you mean Urr, ummm. Double capitalisation there would indicate you're talking to someone called "Umm".
And if you want to get technical it's a death cult. Fudge this life, and everyone in it, the good stuff comes after you're dead, so who cares if you kill babies by the billions, it's an "infinite good":
https://www.youtube.com/watch?v=aUMzYA3XSEc
Abhorrent to anyone sane, and even most of the insane wouldn't agree. But apparently God, the same God who tells you he is real, says he's right.
Because every religion is just the internal monologue of the psychosis of the one "believing" in the voices in their head.
Oh, why the BS on "She"? If you found out I was male (or if you found out I am trans, either male by birth certificate or female, what would change? Then why the hell keep bullshitting on about "She"????
The masculine today was the ungendered originally, and there were terms for male gender and female gender. Which is why "Woman" is "Wo" and "Man". "Man" was neutral and the "Wo" indicated the feminine.
Or do you think that Woman is more than man? Female more than a male? "She" 50% more than a "He"? After all, they have their own special term.
Once upon a time, you had your own gender term and lost it.
How terribly careless.
You don't know who I am, and can only claim, but have obsessed over "She" because you hate women, think them less than women, and have absolutely no clue why the hell you're so full of bile and hatred. So pick the parts of your death cult mythos that "Justify" your toxic personality.
But you pick and choose which bits.
So you're not obeying the word of your god, you're choosing which bits you're going to obey.
Your religion is yours alone and says EVERYTHING about you, whether you understand it or no.
*SNORT*
*LAUGH*
*GIGGLE*
Whooo boy that was a good read! Comments section never fails to entertain!!! Funny how boiling water turning into snow turns into a whos dick is bigger competition.
Hahahaha, where is the love guys?
What IF - You had a large inflated balloon in space with a separate larger balloon around the first and liquid water [urine.?] was injected between the balloons until the gases state formed a thick ice crystal shell around the inner balloon forming a solid skin with a hollow center , could that sphere become a space craft ? could it be pushed into reentry to become clean pure water vapor by friction heat & low pressure ? question s filled with what if flood my mind , What If that's a way water & life can be terrafarmed to other planets ? Or how water & life got on earth ?