"Celebrate the independence of your nation by blowing up a small part of it." -The Simpsons
For those of you who aren't from the U.S. or U.K., this coming Monday is the day that my nation celebrates the birth of its independence. This date, of course, as Aimee Mann will sing to you, is the

How do they work? That sounds like a question for science! (Inspired by Janet Stemwedel's article from 2007, as well as a recent article over at Scientific American.)

You start with three simple ingredients: sulfur, charcoal, and a source of potassium nitrate. Charcoal, in this case, is not the briquettes you use on your grill, which often contain no actual charcoal, but is the carbon residue left behind by organic matter (like wood) once it has been charred (or pyrolyzed), and all the water removed. Potassium nitrate is found in sources like bird droppings or bat guano. Take a mortar and pestle, mix them together, and what you'll get is a fine, black powder.
Gunpowder, in fact. All you need now is some oxygen -- readily found in our atmosphere the potassium nitrate source (see here, and thanks derek @1) -- and a small source of heat. (A match will do.) Put it all together?

Well, you'll get an explosion (and a loud boom), but that's hardly a firework! After all, if you've ever seen one, you know that the four major things that make a good firework are height, size, shape and color.

Physics has something to say about each one of these! The height is the easiest one, so let's start there.

The way you launch a firework is basically the same way you launch a cannonball out of a cannon! You put a "lift charge" in between the actual firework and the bottom of a strong, closed tube/pipe, and ignite it, propelling the firework up.
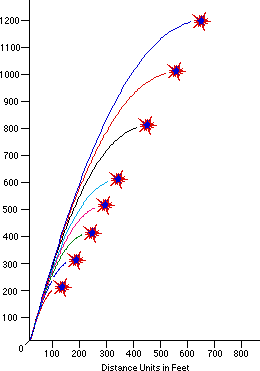
How high you want it to go is dependent only on the initial velocity of your firework, which is almost always larger for bigger fireworks.
A small fireworks show might have 2" (5 cm) to 6" (15 cm) diameter shells being launched, which reach a height of anywhere from 200 to maybe 500 feet (60-150 m). But a very large fireworks show, like the one in New York City, uses fireworks with shells up to two or three feet in diameter (up to nearly a meter), and those fireworks often reach altitudes of well over 1,000 feet (300 meters).
Once the initial launch happens, the fuse on the actual firework itself -- if all goes properly -- is now lit, and burns as it goes up.
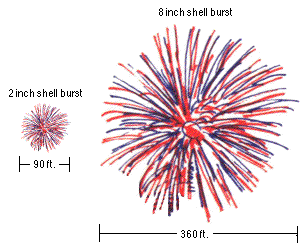
For aesthetic and safety reasons, you launch the larger fireworks to a higher altitude. The physics helps out tremendously with the size of your fireworks as well, because a larger firework requires a larger lift charge! And the amount of the lift charge that you use is sufficient to launch it to the necessary altitudes described above, which is why larger fireworks get launched to higher altitudes.

So long as they're not "duds" (i.e., so long as the fuse ignites and burns properly), they will explode at or near the apex of their flight. The higher ones are usually larger, resulting in, well, aesthetically-pleasing (and again, safer) fireworks displays. But what determines their spectacular shapes that they come in? To find that out, we need to go inside the anatomy of a firework.

Fireworks come in many different styles, but the two important elements, once your firework has been launched into the air with its fuse lit, are the burst charge and the stars.
The burst charge can be as simple as more gunpowder, or it could be a more complicated (or even a multi-stage) explosive. The stars, on the other hand, are what actually go off in many directions, producing the beautiful display. When the fuse burns down to the point where it reaches the burst charge, it ignites!
This ignition, depending on how the firework is put together in the first place, will send the stars off into whatever pattern or direction it was designed for.
It will also reach high enough temperatures to ignite the individual stars. This is where -- for me -- the most interesting part of the fireworks happens. In addition to whatever (optional) propulsion or fuel exists inside these stars, such as the ability to make them spin, rise, or thrust in a random direction, the stars are also the source of the light and color we find in our fireworks.

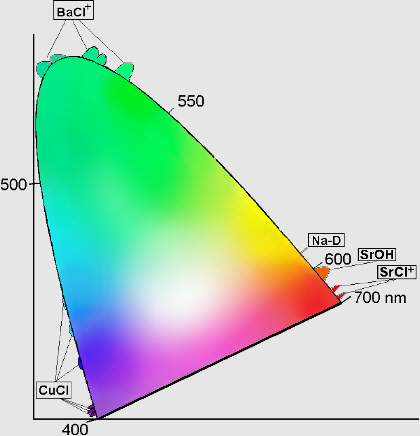
How are these "stars" responsible for color? Although there are some recent advances (covered in excellent detail by Janet), the simplest explanation is that different elements and compounds have different characteristic emission lines. For example, if you take some sodium and heat it up, it emits a characteristic yellow glow, because of its two very narrow emission lines at 588 and 589 nanometers. (You're probably familiar with them from sodium street lamps.)

Well, we have a great variety of elements and compounds that emit a great variety of colors! Different compounds of Barium, Sodium, Copper and Strontium can produce colors covering a huge range of the visible spectrum, as shown below (thanks to this site) in chromaticity space.
And that's how fireworks work, from launch, up to the proper height, to their explosion, to the size, pattern, and color of the spectacular show they put on!

What more can you ask for? Alright, how about the boom sound that accompanies it? Believe it or not, it's the same principle that the sound of lightning -- thunder -- operates on! Update: No, it doesn't!

Just like lightning, the gunpowder explosion superheats the air, causing it to expand and become very rarefied (i.e., have such a low density that it's almost a vacuum). This heating and expansion takes place in a tiny fraction of a second, but it's not the heating and expansion that makes the sound! It's what happens in the instant afterwards. Although this is a reasonable explanation for how lightning makes a sound, fireworks don't go through all of this. It's actually much simpler.
The air from just outside this rarefied area rushes in to fill up this low pressure area. It does so with such great speed that it actually breaks the speed of sound, creating a sonic boom! And that's what makes the "pop" or "boom" sounds that accompanies fireworks. The "blast" from the explosion of gunpowder creates an outward moving pressure wave. At the front of this wave, this pressure reaches very high levels, many times that of the normal atmosphere. The "boom" you hear is simply the rapid change in pressure in the air, which is all that sound -- a pressure wave -- really is. (Thanks to daedalus2u in the comments for catching this egregious mistake!)
And that's the physics of fireworks! Now, go enjoy the show!



Gunpowder doesn't need oxygen, it's basically nitrate plus carbon makes carbonate plus nitrogen (with the sulfur playing a complicated role).
Thank you for that post. I have experimented in the past trying to make my own fireworks. It's been probably about 5 years now since the last time so I was going to re-attempt it this year and this little guide gave me even more of a boost of motivation! You would be surpised how much cool stuff you can get just by combining the cheap knick knack fireworks. Use sparklers for explosions/lift-off charges, add crackering balls and spakler powder with weakened areas in the packaging to control where it shoots out. Definitely more fun watching something you made blow up than your average firework. Plus the fact you pretty much have no idea what it will do keeps it suspensful lol.
Ethan, I think you want to look a little more carefully at the details of the production of acoustic waves from sudden expansions of gases. The energy in the positive pressure wave from the initial expansion is usually a lot more than in the negative pressure from the rarefaction. The initial expansion from high pressure into one atmosphere air only stops when the expanding gas has expanded to below one atmosphere. The energy contained in the sub-atmospheric gas which then drives the collapse is considerably smaller than in the initial high pressure gas before expansion.
Shocks in air only occur when there are shock waves (usually). Generally you don't get them from sub-sonic flows (like the deflagrations in fireworks). Lightning is fast enough that it can be shocks.
This is different than cavitation in liquids where you do get shocks when the bubbles collapse, but liquids are pretty incompressible so when the bubbles collapse (to a point), the energy of the collapsing liquid can't go anywhere so it goes into a shock.
Cody, unless you really know what you are doing (in which case you should know exactly what is going to happen) and take the proper precautions and use the proper safety equipment (gloves, apron, eye and face protection), it is extremely dangerous to do what you are suggesting. Many fireworks are not designed to be disassembled and cannot be disassembled safely. Some of those mixtures can be ignited by static electricity, by mechanical damage, by shock, by contact with water, or even spontaneously.
Many of the compounds are quite toxic before ignition (for example perchlorate is an iodine mimic). Barium, copper and arsenic are pretty toxic too. Usually fireworks don't list what they contain, so you might expose yourself to toxic things without knowing it.
Linked.
I borrowed a photo (my bandwidth). If you object I'll remove it.
I don't care about the color or the height. I like the ones with the big bright flash followed by an enormous blast. It's not about the beauty. It's the violence. Peace... God bless...
daedalus2u,
You are right; I am wrong! I have a pretty severe correction to go back and make. Thanks for the catch!
-Ethan
Ethan, you are welcome, that is what we scientists do for each other. I am always happy to help.
Often in movies of explosions in humid regions, you can see the condensation in the rarefaction wave that follows the compression wave. The adiabatic expansion in the rarefaction wave has dropped the temperature below the dew point and water condenses as fog, only to evaporate when the pressure goes back up.
I pretty much second all of what Daedalus is saying, gloves, goggles/ full face shield, heavy full leather jacket would be an absolute minimum unless you think scars from burning, and lack of eyes is a good thing.
I'm not quite sure what the microburst illustration is doing here. That last pic is the "why microbursts are a bad thing for planes near landing" illustration. In short, a sudden, severe downdraft is a BAD thing when you are only a hundred or so feet from the ground.
@daedalus2u
Thanks for the warning. We keet it a fairly sterile environment whenever we do it. Definitely want gloves, airmasks, goggles, etc. One of the main sources of the actuall powder comes from the sparklers. You buy the metal ones and break the powder off and slowly grind it down in small amounts. You gotta be patient and slow not too ignite it from pure friction. Plus doing it in smaller amounts keeps any accidental ignition to be rather benign. It can easily take 3-4 hours just to make one with 4-5 people breaking down the fireworks.
For effects the strobe flashers & crackering balls work rather well as the packaging if very minimal. You learn pretty quickly which ones are worth breaking into and which aren't. You can also cut into reloadable canisters (artillery shells) relatively easily but you usually don't need them.
Safety is definitely a major concern but at the same time the small element of danger gives you a decent adrenaline rush :)
I have helped set off professional displays and while I always had a decent guess as how they worked I never knew exactly how they were put together. It was especially cool to learn what compounds make the color.
Since everyone seems to be correcting you I thought I'd jump in too :D. Gunpowder only makes a flash and a fzzzt sound when you put a match to it as described. It only explodes and makes a boom when you contain it in something and the pressure caused by the chemical reaction causes it to push out and rupture the container. Although I guess this post comes close enough to how to make a homemade bomb w/o throwing that in there.
@Cody, I agree with daedalus2u, I shot public displays for 20 years and even as careful as we were we still had some close calls. Even as careful as you may be there are combinations of fuels and oxidizers that you may obtain that are quite dangerous. Some may even detonate simply by being placed together with no ignition source. I would suggest that given your obvious curiosity that you obtain proper materials and instruction to make your own fireworks safely. I would also look into volunteering with whatever local group that puts on your public fireworks display. That way you will get to get close and personal with the big shells as well as meet people who have the knowledge to perform these activities somewhat safely. Always keep in mind that fireworks are NEVER safe but you can minimize the danger with proper care. If you really want to make your own there are ways in most states but it is a lot of effort to get the permits and space to do so. Please don't hurt yourself.
Cody, I never took fireworks apart, I made them myself from scratch from known components when I was young and foolish. I was very âcarefulâ and never had any âclose callsâ, but did do things that if there had been a âclose callâ at the wrong time it would have killed or maimed me. I would never do now what I did then knowing what I do know now, or let my children do what my father let me do.
Grinding any mixture of oxidizer and fuel is extremely dangerous unless done wet (it can be dangerous even then). It isn't something that can safely be done dry, no matter how âgentleâ you are trying to be. The minimum energy to break aggregates can be enough to ignite them. Static electricity on non-conductive containers (like plastic) can cause ignition too, even if the mixtures themselves are electrically conductive (usually they are when in condensed form, but when dispersed in air, the air-powder mixture is not electrically conductive).
The cost of the minimum safety equipment you need is (probably) going to be considerably more than the value of what you are doing.
If you want a âthrillâ from doing something dangerous, find something that has a redeeming social value, like being a volunteer firefighter. Or is safer and more predictable, like tight rope walking.
Lol - Wicked info, thanks Ethan. Ithink you may need to rethink the comment "For those of you who aren't from the U.S. or U.K., this coming Monday is the day that my nation celebrates the birth of it's independence" - we don't celebrate it in the UK, or even really acknowledge itâ¦
Typo in first sentence.
Aside from the consistently superior subject matter, one of the things I appreciate most about the ScienceBlogs is that it's one of the few blogs I can think of that, when an error or a needed correction is pointed out in Comments, the host acknowledges the assistance and makes the correction, instead of acting offended. This, alone, sets ScienceBlogs apart. Kudos to you, sir, and to the knowledgeable readers.