"They credited us with the birth of that sort of heavy metal thing. Well, if that's the case, there should be an immediate abortion." -Ginger Baker
As hard as it may be to believe, take a look outside. I don't mean a glance, I mean to take a real look. At all the things there are to see -- the rocks, trees, mountains, skies, clouds, Sun, water, and everything alive -- all of it.
Now ask yourself, "what's it made of?"
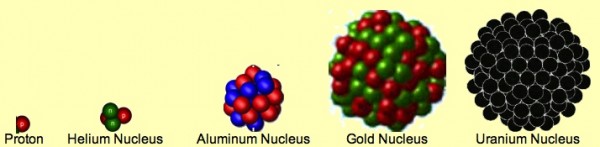
At a fundamental level -- like everything else you know of -- everything on Earth is made of atoms. Oxygen, Hydrogen, Carbon, Nitrogen, Calcium, Iron, Gold, etc., all the elements of the Universe combine together in a huge variety of ways to create everything we know of an observe in the Universe. When we peer inside of them, we can find the very thing that gives each and ever atom its special properties.
Unbelievably, it's simply the number of protons in each atom's nucleus. And this huge variety of things that we have in our world only comes about because of the huge variety and abundance of these elements, from Hydrogen to Uranium and beyond.
But these elements haven't always been around. And they certainly haven't been around for all that long in the abundances they're found in today.
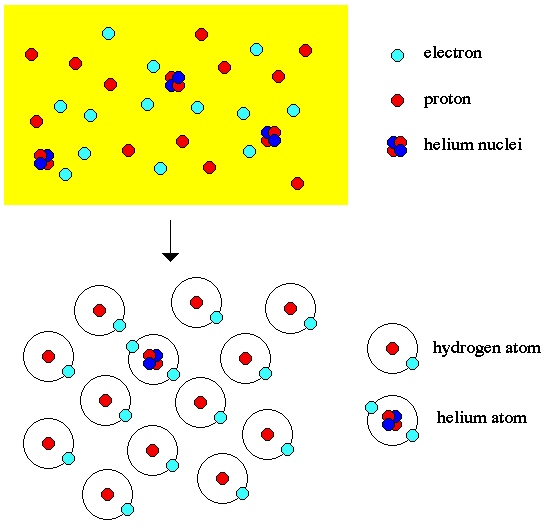
In fact, just a few minutes after the big bang, the Universe has cooled to low enough temperatures that it's done undergoing all the nuclear reactions it can possibly undergo. And at that point, we can take a look at what all the elements in the Universe are.
Perhaps surprisingly, the Universe is made up of (by mass) about 76% Hydrogen, 24% Helium, and less than 0.0000001% of everything else, combined. The Universe has no problem cooling and forming neutral atoms after this, but with just Hydrogen, Helium, and minuscule, trace amounts of everything else, you've got to wonder at what we have today.
In other words, where does all this come from?
How we made all the elements we have today is the same way we're making them right now: in stars.
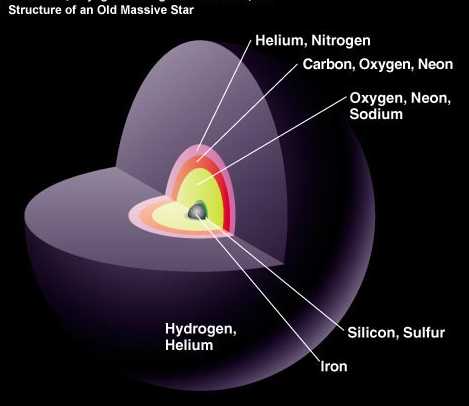
Our Sun, like the vast majority of stars, is fusing hydrogen into helium, and that's what's powering it. But the heaviest, most massive stars burn through their fuel far more quickly.
And once they burn through their hydrogen, they fuse that helium into carbon, and then into nitrogen, oxygen, neon, and sodium, and then into silicon and sulfur, and then into iron, nickel, cobalt and copper.
The ones that make it this far are anywhere from eight times the mass of our Sun up to hundreds of times as massive. While our Sun takes somewhere in excess of ten billion years to burn through all of its fuel, these more massive stars take anywhere from tens of millions of years down to just tens of thousands of years before they exhaust the fuel in their core! And then, what happens next is simply spectacular.
The star in question goes supernova, where enough energy is released to form all the elements in the Universe, and to form them in great abundance.
And, as you can see in the video above, these elements are released out into the Universe. If you're a hydrogen/helium "purist", you might say that they pollute the Universe. But if you're all in favor of these heavy metals and elements throughout space -- like me -- you'd say that they enrich the Universe.
In some regions, where you had lots of high-mass stars in the past -- particularly if you've had multiple generations of stars -- you'd expect that you'd wind up with plenty of metals. Perhaps, in fact, that's the picture you have in your head when you think about our Sun. After all, when we take a look at our Sun, we see an incredible number of spectral absorption lines, surefire indications of heavy elements!
Unlike the "pristine" Universe, our solar neighborhood is enriched, with about 2% of all the elements found in it heavier than hydrogen or helium. Our Sun has been through at least two previous generations of stars that have formed, burned their fuel, died, and enriched their region of space. But it is by no means one of the most enriched regions of the Universe, or even of our own galaxy.
Where do we look for that?
It's the centers of the most massive galaxies -- the brightest, most active, violent regions known in the Universe -- that possess the greatest abundances of elements heavier than helium, collectively known as metals.
Galaxies can form stars just 50-100 million years after the Big Bang in our Universe, and can go through not just one or two but many generations -- in the most massive, rich galaxies -- before we ever see the light from them.
Which is why every time I see a report like this (or this):
In a surprising find, scientists have detected carbon much earlier in the Universe's history than previously thought,
I'm completely baffled. Because unless they mean "in the 1920s" when they say "previously," that's not what we think at all!
This is TN J0924-2201, the most distant radio galaxy ever discovered. (At a redshift, z, of 5.19. For redshifts, higher numbers mean the Universe was younger and the object is farther away.) And this is the scientific paper that was written about it. But is this a surprise that there are so many heavy metals in this galaxy? Let's quote from the article itself:
there is no redshift evolution of... metallicity in the redshift range of 2.0 < z < 4.5. Using near-infrared spectroscopic observations... Jiang et al. (2007) found no strong evolution of... metallicity up to z ∼ 6. Recently, Juarez et al. (2009)... found that the metallicity is very high even in quasars at z ∼ 6. These results indicate that the major epoch of chemical evolution in AGNs is at z > 6.
So don't be fooled; the Universe is well-known to be rich, full-of-metals, and highly evolved just a few hundred million years after the Big Bang, or when it's only 5% of its current age!
For example, take a look at this "baby" galaxy.
This galaxy, a mere 700 million years old, is so redshifted that even the light coming from it -- most of which is blue and ultraviolet in color -- has been shifted out of the visible part of the spectrum! Yet this galaxy is not only eight times the mass of the Milky Way, but is even richer in these heavy elements than our Sun is!
And yet, we know that -- at some point in the past -- the truly first stars formed, made up only of hydrogen and helium. Well, where did that happen? Our only choice is to keep looking back.
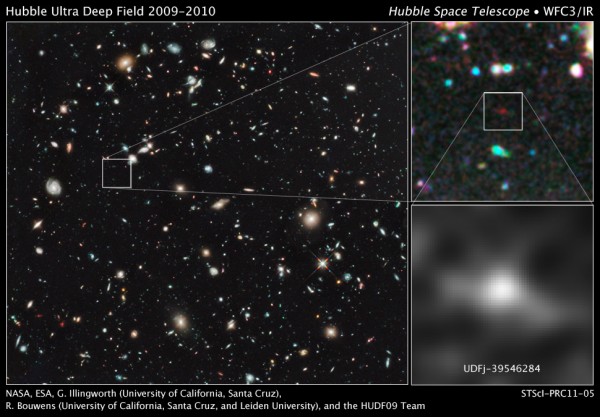
This is the current record-holder for most distant galaxy ever: UDFj-39546284, from when the Universe was only 480 million years old, or 3.5% of its current age!
This galaxy is a small collection of hot, blue stars, with not even 1% of the mass of the Milky Way! Is this where we were forming the first stars? Or is this galaxy even typical of the galaxies that are out there at this early stage in the Universe?
Our best theories tell us that we wouldn't be surprised if galaxies as far back as this one were abundant, rich in metals, and -- in many cases -- of comparable masses to our own Milky Way, already. But at some point, some distant galaxy was first. And we want to know where that was, and when that was.
At this point in time, there's only one plan in the works to find that out.
Add just one more reason to the list of why we need the James Webb Space Telescope!
So until we get there, don't be surprised that the distant Universe is full of heavy metals, evolved stars, or massive galaxies. The Universe is a place where everything we know can not only happen, it happens fast. It does make you wonder, though, just how long ago -- under the right conditions -- planets, and even life, could have formed!
- Log in to post comments










I hope that was a Gamma Ray reference!
www.youtube.com/watch?v=uve1QZ9izK4
Looking at the VFI Atomic Radii chart, I spotted something odd: What's the deal with Radon (86-Rn?) Elements within a group tend to get larger with increasing atomic number. Looking down the Noble Gases column, the trend is followed nicely until we're surprised by tiny Radon being way smaller than huge Xenon.
Polonium seems a bit of an oddball too, though not as extreme.
Poking around elsewhere, I read that the van der Waals radius of Radon is ten or fifteen percent LARGER than Xenon. So maybe the question is, what's the deal with this chart rather than what's the deal with Radon.
Any simple explanation for this?
"It's the centers of the most massive galaxies -- the brightest, most active, violent regions known in the Universe -- that possess the greatest abundances of elements heavier than helium, collectively known as metals."
So you are saying we found a galactic moshpit?
Those stars in the early universe must have formed by contraction. The only known force to withstand expansion is gravity.
There should not be that much carbon if the universe is expanding.
The gasses anywhere within this early universe should contain less than 0.0000001% of metals [carbon and higher elements].
The problem with abundances of elements heavier than helium, is that it takes time. Even very big stars need some time for fusion. And it would be very difficult to see through this "mist" of 99.9999999% of other neutral atoms around that observed star.
Sorry Ethan,
but in my view this is another result showing that Ludwig Boltzmann made an error.
Only if one thinks that a thing is the sum of its parts. No an easily held phisolophical position. Especially with the current advances in molecular biology!
Ok with the nuclear physics and astrophysics, but how can a thinking person actually blame Ginger Baker for heavy metal?
A few thoughts (for what is worth)
The further we explore the more we will find all kinds of stars and galaxies including ones thought to be billions of years old even at high redshifts. At some point, the Big Bang theory will have to give in when the data keeps challenging it. You can't simply assume that "it is all right that there is mature galaxies only millions of years after the Big Bang". More so that with technology improving we will see a more diverse variety of galaxy types at such distances (another prediction of mine).
What kind of data would falsify the Big Bang theory?
Your post lead me to a question I hope you'll answer:
Can photons take on any frequency?
My question isn't really about lower and upper bounds, but let's start there.
Upper bound: Since the energy of a photon is E = hv and since the Universe presumably doesn't have infinite energy, there clearly must be an upper bound on a photon's frequency. Do we know what this bound is?
Lower bound: I have no information to guide me here except to say that a photon with a frequency of 0 probably can't be considered a photon. Do we have any better lower bound?
And now to my real question. A photon can be created by an electron in an atom falling from a high energy state to a low energy state. But these states and their energy levels are discrete and therefore no matter how many types of atoms or molecules you have, the photons thus released can only cover a finite, discrete range of frequencies.
OK, but there are other ways to make photons. Such as nuclear reactions. I'm not too famaliar with these reactions, so can you help me here as to whether they can produce a photon of any desired frequency?
Lastly we have the expansion of the Universe. Photons sent off when the Universe was young have had their frequency reduced due to spacetime expansion. Does this mean these photon's frequency, as recieved here on Earth, now cover all infinite types of frequencies? I personally don't see how.
Here's hoping you will answer my question! :-)
It's the centers of the most massive galaxies brightest, most active, violent regions known in the Universe. Lastly we have the expansion of the Universe. Photons sent off when the Universe was young have had their frequency reduced due to spacetime expansion. Does this mean these photon's frequency, as recieved here on Earth, now cover all infinite types of frequencies? veysen karannınid dizelerinden fıÅkınard bir düÅencelere vardır. heavy metal universe vayydiyereke sonulçalrımÅtır. iÅte burdaruların neticelere varbmisene igneoyası ismekkurslarındoan yararlanarak bir alie bütçesinee kazanç kapısınıı açmalızısınmz.
GWAR's guitarist is out there somewhere...spewing fluids on someone else's galactic core...
#9: Dear igneoyas1, kindly translate your answer below to English:
Photons sent off when the Universe was young have had their frequency reduced due to spacetime expansion. Does this mean these photon's frequency, as recieved here on Earth, now cover all infinite types of frequencies? veysen karannınid dizelerinden fıÅkınard bir düÅencelere vardır. heavy metal universe vayydiyereke sonulçalrımÅtır. iÅte burdaruların neticelere varbmisene igneoyası ismekkurslarındoan yararlanarak bir alie bütçesinee kazanç kapısınıı açmalızısınmz.
I see no mention of adamantium in your article. That does not comport well with your picture up there Ethan!
Do the farthest galaxies look just like the nearest galaxies, in terms of metal, e.g. carbon and other things, and all observable details? I assume yes.
Specifically, is there any observably different from a very distant galaxy and a near one? I assume no.
And what exact differentiators will the JWST be able to focus on? (e.g. any chance to determine the age of white dwarfs of a globular cluster in a distant galaxy? or find a metal free star?)
If there is no observational difference; then it stretches credibility to believe in a big bang evolutionary interpretation (despite all the "apparents" from Hubble's velocity on to inflation).
I mean an old galaxy is a fossil. And an evolutionary expectation, assuming big bang is correct, is that an astronomical fossil must somehow be different from a galaxy of today.
"I assume yes."
They're not. Mostly metal-poor.
"Specifically, is there any observably different from a very distant galaxy and a near one"
Different what?
"If there is no observational difference;"
You had to assume that, though. Assume that you're wrong.
@13: I think if you google "distant galaxies," your question would be answered in favor of the Big Bang scenario. It's not so much the composition of the early galaxies that's different as is the size and formation of them -- they're tiny, compared to the Milky Way or Andromeda -- let alone the elliptical giants -- and resemble scrap stellar fields like the Magellanic Clouds more than they do other formations.
The surprise is the amount of heavier elements they contain. But rather than disproving the Big Bang scenario, this goes a long way toward proving the existence of ancient supergiant stars, perhaps with dark matter cores, that formed and supernovaed within a million years or so of the Creation Event.
The halo of globular clusters around the Milky Way, and globular clusters in general, are remarkably ancient and heavy element/metal free. As a matter of fact, before the date of the Big Bang was extended back, there was a well-publicized dispute between Steady Statists and the astronomer Carl Sandage about whether these formations pre-dated, and hence disproved, "the Big Bang," (they didn't and don't).
However -- from what I understand (and my understanding is often not perfect) -- chemical analysis of extremely early galaxies has turned up more heavy elements -- including metals, I should think -- than previously expected. But as I pointed out above, this discovery happened at the same time that new findings about dawning star formation occurred, thus one cancels the other out, so far as providing an escape from the Big Bang scenario.
It's why that Arecibo message to a globular cluster was a dumb idea. Although there are a lot of star systems in the beam, none of them will have enough metals to make a solid world, solid enough to hold an RF antenna.
Still, it made good copy at the time...
Also, if any passing star with intelligent life in the vicinity happened to get in the way of the transmission...
Which could be the source of the "WOW!" (speaking of Wow) signal picked up back in the '70s.... We happened to be in the right place at the right time, for a transitory directed message.
Wow
You too quickly assume that I am wrong;
my base assumption is agreement with Ethan's summary astronomy OBSERVATIONS:
"the Universe is... full-of-metals, and highly evolved just a few hundred million years after the Big Bang, or when it's only 5% of its current age!... best theories tell us that... galaxies as far back as this one were abundant.. in metals, and.. of comparable masses to our own Milky Way, already."
Ethan theorizes, "But at some point, some distant galaxy was first. And we want to know where that was, and when that was... there's only one plan in the works to find that out... we need the James Webb Space Telescope! So until we get there (JWST), don't be surprised that the distant Universe is full of heavy metals, evolved stars, or massive galaxies." But my question is only about OBSERVATION.
Clearly, Ethan means that ONLY heavy metal galaxies have been observed.
So, my question of @13 stands. Let me clarify further.
What is different (in observation not theory) about a galaxy 800 million years after the hypothesized big bang and a galaxy of today?
Remember our sun goes around the Milky Way galaxy once every 250 million years. So a galaxy of "5% of universe's current age" i.e. 800 million years and of "comparable masses to our own Milky Way" has had the short time of only 3 galaxtic cycles. With such a short time to develop; I would expect some observable differences. Yes, yes.
Ethan has clearly said that both fossil and present day galaxies are heavy metal, i.e. "the distant Universe is full of heavy metals, evolved stars, or massive galaxies."
So, is there a spectral or structural difference OBSERVABLE (with today's telescopes) between an early galaxy and a present day galaxy? Or are all of the astronomical fossils galaxies essentially identicle to some present day galaxy?
Wow, please STOP and THINK; stay with my question by sticking with OBSERVATION differences; don't drift to theoretical differences.
"Jiang et al. (2007) found no strong evolution of... metallicity up to z â¼ 6."
...this sentence should read as z=0 [today] up to z~6.
So the universe from today up to z=6 shows no significant change in metallicity. Therefore all change must appear from z=1100 to z=6. Which is a relatively very short period, regardless of the differences in z here.
If a BLR [broad line region spectrum] is representative of a in general higher metallicity, there also must take place metallic ejections or a mixing with lower metallicity gasses later [to match the metallicities observed in todays local massive galaxies like the Milky Way].
This may prove very difficult because recent publications show that an upward revision of metallicity is needed also in some of the most metal-poor small dwarf galaxies known, like UM151 and UM408. Merging gasses won't help much here.
I think you cannot explain away this annoying discrepancy in regard to an 'expanding' universe.
See also recent news about "Population explosion in dwarf galaxies". A team of astronomers led by Arjen van der Wel of the Max Planck Institute for Astronomy (MPIA) in Heidelberg.
It is easy to explain the forming of this explosion of star forming dwarfs in an early contracting universe. No need for dark matter in that way.
I love that solar spectrum from the NSO. The image of course is faked - synthesized from Fourier Transform spectrometer data and colored in by computer, meant to mimic older photographic color spectra, and actually extending from the mid-IR to the UV an not the red to violet in the image. About 10 years ago I stumbled across a photo positive solar spectrum produced by Ron Giovanelli - the original film of a reproduction that used to hang on the wall at the NSO.
I'd like to see the JWST fly - the instruments should fill a huge gap between the millimeter instruments like Planck, Herschel, and the now defunct Spitzer and the more typical near IR to UV instruments.
Regarding my comment @19, Ethan's post of today
Nov 14, 2011 Found: The First Atoms In The Universe!!!
Answers my question.
Gas cloud at 12 billion lightyears is the sufficient missing link that I ask for. I got it. Thus I must accept the big bang theory. I don't have another, in my mind, necessary big picture missing piece of evidence. See my comment @13 in Ethan;s Nov 14 post.
Not to derail this thread (since this newsclip could distantly refer to "metalicity" in nearby galaxies), but --
You thought climate change was all we had to worry about:
http://news.yahoo.com/why-milky-way-may-facing-midlife-crisis-210401548…
Yes, as our galaxy does the do-si-do in its approach to scrumptious Andromeda, it may need some cosmic viagra to complete the act -- because it's getting middle-aged.