“...the magnificence of the Universe as revealed by modern science might be able to draw forth reserves of reverence and awe hardly tapped by the conventional faiths.” -Carl Sagan
As many of you know, I'm fortunate enough to live in a city that values science and scientific knowledge so highly that the our local news station, KGW, routinely brings on scientists to talk about the lastest developments in our endeavors to understand the Universe around us. Just last week, I was invited to share five wonderful minutes of airtime with the viewers of not just Portland, OR, but (thanks to the web) all over the globe.
What he said to me after that was something that I've heard often, but that I've never addressed here. He said to me,
You know, I can't really think about something like that for too long. It makes my head hurt, and it starts to freak me out a little bit.
It's a sentiment that is far from universal, but one that I think I understand, because when I think about it, I freak out, too.
It's part of what drew me to this field in the first place, honestly. We are born into this world barely aware of what we are, and completely unaware of the size, scope, and history of the Universe that brought us into existence.
And when we first hear about the actual scales and ages of things in the Universe, it can be terrifying.
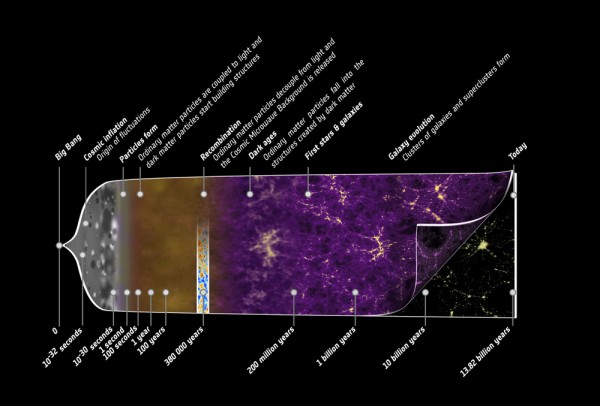
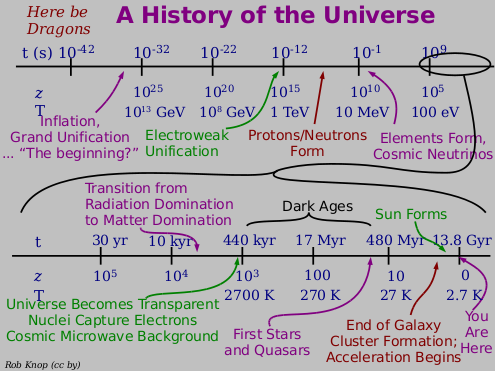
The entire Universe is 13.8 billion years old, and the Earth and Sun have been around for about a third of that. If you were to compress the entire history of the Universe into a single calendar year, I would have been born at 11:59 and 59.9 seconds PM on December 31st, and in fact the first homo sapiens would only have appeared for the final seven minutes!
For all the time that the Universe has been around, that the stars have been shining, that the planets have been orbiting, our own lifetimes are small, minuscule, and, at least as far as the rest of the Universe is concerned, cosmically insignificant. If you were to compare your lifespan -- even if you lived 100 years -- to the age of the Universe, that's the same as if you compared 23 seconds to your entire life. In other words, your time in this Universe is very, very short.
And if instead of time, you start pondering size, the story is just as awe-inspiring.
 Image credit: docstoc, via user HC120712222550 of http://www.docstoc.com/ (public domain).
Image credit: docstoc, via user HC120712222550 of http://www.docstoc.com/ (public domain).
As humans, we're on the order of a meter in size, some ten orders of magnitude larger than the atoms that make us up. A typical human is a little under two meters tall; a typical atom is a little under two Ångströms in diameter. But ten orders of magnitude is tremendous.
A giant star like Arcturus, some 25 times the diameter of our Sun, is around ten orders of magnitude the size of a human being. And to the Universe, that's still nothing! From Arcturus, if we scale up another ten orders of magnitude, that's what it takes to get a typical small galaxy like -- not Andromeda -- but the small satellite galaxy visible just below the large Andromeda galaxy!
 Image credit: © Fabian Neyer of http://www.starpointing.com/.
Image credit: © Fabian Neyer of http://www.starpointing.com/.
And a galaxy like this is made up of more than a billion Arcturus-massed objects. And to go up another ten orders of magnitude... well, you can't. The observable Universe -- all of the galaxies, all of the stars, planets, lifeforms and atoms -- is "only" some 1027 meters in diameter, or just seven orders of magnitude larger than the diameter of a small galaxy.
In other words, you are small. The difference between an atom and you, in size, if you cubed it, is about the difference in size between you and the Universe.
But if you look at energy, or mass (which is where most of your energy is), you get a different perspective.
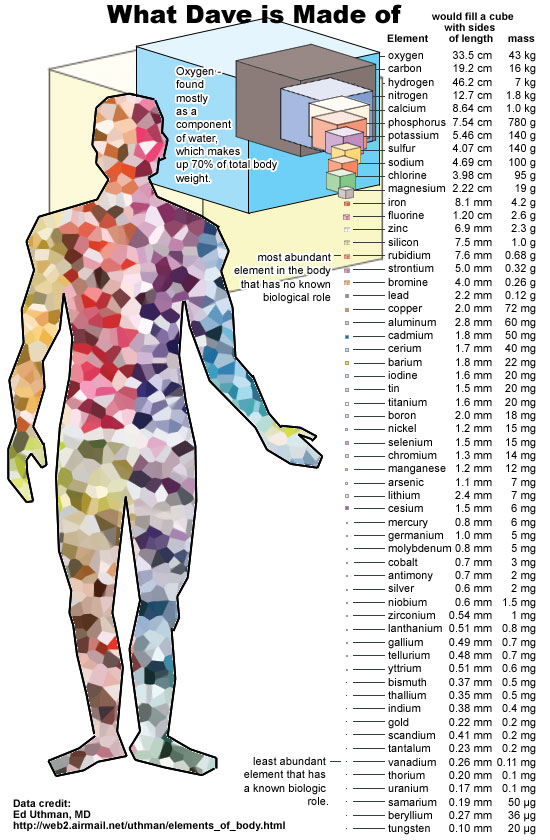 Image credit: Ed Uthman, MD, of http://web2.airmail.net/uthman/elements_of_body.html.
Image credit: Ed Uthman, MD, of http://web2.airmail.net/uthman/elements_of_body.html.
Small though you may be, ten orders of magnitude larger than an atom, you yourself are actually an entire Universe of atoms, with more than 1027 atoms making up your body! (This makes a little bit of sense, since you're the height of 1010 atoms, and you're a three-dimensional object.) But this is highly disproportional to the bulk of the Universe.
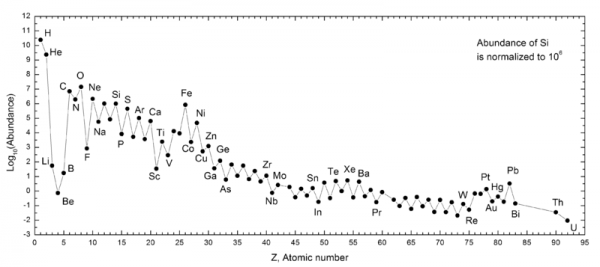
You see, only 1% of the Universe, by mass, is made up of elements other than hydrogen or helium. And yet, you are 90% "other stuff," and only about 10% hydrogen.
And while a Sun-like star might be about 1027 times the mass of a person, going up another factor of 1027 would encompass more than the entire Universe.
You see, there's no denying that we're here for only a short time. Even if we found a way for humans to live 1,000 years, or even a million years, it would still pale in comparison to the age of the Universe. Coming to grips with our own transience and brevity in this Universe is something we all have the opportunity to do; whether we do or not won't change that fact.
There's no denying our smallness in scale, either, as the largest scales in the Universe are blissfully unaware of the entirety of all the goings-on ever to take place on our world. I understand the simultaneous awe and terror about this fact.
But as far as what we're made up of -- our energy, our mass -- we are absolutely privileged. Generations of stars lived and died, fusing a Universe that was once 99.999999% hydrogen and helium into a tiny amount of heavy elements, which formed, after billions of years, into the stars and planets that allowed life as we know it to exist.
We are so little on our own, and so insignificant compared to the ancient, vast and incredibly massive Universe.
And yet, we're here, we exist, and we get to bask in our existence however we so choose. Everything we are, everything we have, everything we see, is the ultimate free gift of the Universe to us.
And I can think of no better way to use that gift than to try and understand it, and by understanding it, perhaps understanding ourselves a little bit better.
- Log in to post comments






Well said :-)
Good stuff Ethan. I walk outside on a clear night and raise my binoculars to the Milky Way, and even that gives me that feeling of cosmic awe.
"...Everything we are, everything we have, everything we see, is the ultimate free gift of the Universe to us. And I can think of no better way to use that gift than to try and understand it..."
I couldn't agree more. Yeah, well said.
Excellent article, Ethan, thanks. As for the last four lines - awesome.
That's a wonderful post, Ethan!
I love how it sort of mirrors my own love of chemistry, and what drew me in in those first few weeks of Junior year chemistry in high school. I was trying to get the teacher to be more precise (and accurate as well) regarding WHERE EXACTLY is that damn electron in the orbital, and he gave me reading to do on my own. Once i learned more about electrons, and smaller fundamental particles, it blew my mind regarding how incredibly small these things were. I was hooked, and i read everything our small town library had in a matter of weeks. The constrast between the smallness of a quark or a gluon and the size of the entire universe still sometimes keeps me up at night.
The fact that i ended up doing organic chemistry for a living was mainly because i found the lab work in that section the most satisfying, but my favorite classes and chemistry "hobbies" if you will will always be quantum and where chemistry most strongly overlaps with physics :)
Can we have your liver, then?
Great post! As a youth, I spent hours losing myself in photos of deep space from Asimov on Astronomy and the like. I was convinced from reading sci-fi by Arthur C. Clarke and Larry Niven that our planet would make some technological or evolutionary leap (or we'd receive some space-traveling deus ex machina from kind but inscrutable aliens) which would give us the ability to explore the reaches of the galaxy. Sigh.
Ethan, I am curious if you have any opinion regarding Lee Smolin's incipient model of time, and if it has any credence or value for you. Thanks so much!
We may be small, but our ability to see and understand the universe, though incomplete, is awesome.
Such a beautiful post - gave me chills! Thank you!
a little bit of nit picking about hydrogen atom size:
the Bohr radius is 0.529 Å, so a hydrogen atom is about 1 Å in diamter. a hyrdrogen molecule will be about 2 Å.
If I recall correctly another of your posts about the smallest possible size of the whole universe, given the remarkable flatness of the visible universe, you do have those extra three orders of magnitude to increase from the small galaxy. And probably then some.
Why would anyone assume that a brain that evolved as a survival strategy within the selection confines of the planet Earth would be endowed with the intellectual acumen to 'understand' the 'universe' or 'reality'? All understanding is delusion.
Good post. But Animaniacs anticipated you 20 years ago with Yakko's Universe Song: https://www.youtube.com/watch?v=f_J5rBxeTIk
It's a great big universe,
and we're all really puny,
just tiny little specks,
about the size of Mickey Rooney.
It's big and black and inky,
and we're all really dinky,
it's a big universe,
and we're not.
To understand yourself, look outside yourself. To understand the universe, look inside.
Concur
"The entire Universe is 13.8 billion years old, and the Earth and Sun have been around for a third of that." So if the
the entire 13.8 billions years was compressed to 6 days,
then the sun was made on the fourth day. Seems Iike
have read that somewhere before.
Genesis 1:14
Beautiful post. Thank you Ethan.
Uh, the stars were after the earth, according to Genesis.
Of course, even within that same book there's a second account which disagrees with the first.
But if you want to look at proof by accordance with reality as shown by science, then you should look at the Hindu faith.
Brhama year is about 4.5 billion years.
Earth is about 4.5 billion years old and the universe is about three times that.
Beats a guess of less than 6000 for the universe, but around 6000 for the earth itself, doesn't it?
Good stuff, gets me pumped for you to finish your book so I can buy it. Will you be selling signed copies with moustache trimmings sprinkled inside the pages?
"All understanding is delusion."
That has now taken the number one spot for the dumbest thing I've ever heard in my entire life. Larry the Cable Guy, you have been demoted.
Is there a general table or calculation about how much elements will a star produce for a given lifetime in comparison with it's mass or some other property. Or when it goes nova. i.e. how much carbon will a 1 solar mass star eject when it dies?
I looked at metalicity under wiki, but it seems to give a very general calculation and it mostly deals with H/Fe content.
Thank you
Wonderful. Thanks for this!
Ethan: Nicely done; you're starting to sound like Carl Sagan 2.0, which is exactly what we need today. And after hearing your voice on TV, I'll hear it in my mind's ear when I read your stuff. Making the full media transition to Sagan 2.0 might call for a slightly more conventional appearance to reach a mass audience, but either way is good.
I don't get (except in an abstract almost "clinical" sense) how people can be terrified of the size and scale and scope of the universe. For me it's awe-inspiring, always has been, always will be. There might be a historical parallel with religious attitudes toward deities: the difference between "God-fearing" and "God-loving." At root, both seem to relate to whether someone feels at home in the world and in their own existence. If "yes," then there's a sense of connectedness to the greater whole. If "no," then there's a sense of being alienated from it: either as an insignificant dust spec, or as a sinner in the eyes of the deity.
The tendency of humans to personalize or personify objects around them, is well known. Thus we get the historic personification of an awesome universe as the presence of a deity that is in some way concerned with us. Even automobile stylists make routine use of personification by designing the fronts of cars to have visual cues that suggest faces with various expressions (think of them as similar to emoticons).
Meaning and purpose don't exist in the external world, but do exist in the human mind, which is after all a part of nature. So we can certainly say that intelligent life as we know it has (a sense of) meaning and purpose.
Western cultures have lived with monotheism for a couple of thousand years. But it's only been within the last hundred years that we've begun to appreciate the true extent of the universe, and the complexity and diversity of the objects in it. Where we are today with this, is where ancient peoples, such as the Jews and the early Christians, were when contemplating their newly-monotheistic view of divinity.
We have only begun to see the effects this will have on our culture. And we, by which I mean all of us who are conversant with cosmology to any degree, have an opportunity and also a responsibility, to help shape the emerging worldview.
Consider what happened to monotheism as it was utilized by empires for the sake of worldly power: original teachings that exhorted humans toward peace were somehow twisted into rationales for mass brutality. (For that matter, any philosophy that attracts a mass following can be used as a convenient rationale for brutality, see also Marxism in the hands of Stalin.)
Could something similar happen to the modern science-based view of the universe? Yes, we would be fools to think our present emerging worldview exempt from the historic pattern. Can that be prevented? Yes, if we're willing to grapple with the philosophical implications of science, and be proactive in shaping the culture.
"Could something similar happen to the modern science-based view of the universe?"
What similarities would be done with the justification of science?
We have lysenkoism and the current AGW denial, both *proclaiming* they are science-led, but they aren't doing science.
Science doesn't say WHY you should do something like religion or politics say you should do something, it only tries to say what the consequences of it would be.
And that won't lead to similar abuses. Because it gives no justification in and of itself, only consequence. Unlike religion whose only purpose was to say why you should do X and not Y and justified it on basically "I said so".
Couched in religion, it becomes "Because God Said So".
Couched in science, it becomes "Or Z will happen".
If it doesn't happen, then "Your assertion is wrong". If that happens with religion (e.g. End Of The World Is Nigh), it becomes "He changed his mind, but he'll get you next time, Gadget".
SL, #18, not readily. There are simulations and assumptions for stellar evolution and they will result in just such a table.
But it's not really out there any more, since the purpose of such calculations are for the determination of the age of the universe, and that level of discourse is pretty ancient and other things are much more reliable.
I.e. the universe can't be older than any star you see in it.
The age of the star is based on calculations from stellar evolution. Which are also used to find the ages of open and globular clusters.
But calculations for the abundance of elements are done, and Ethan alluded to that in the recent thread about how the standard model is complete enough to describe reliably the current universe ***including abundances of the elements***.
@ Wow
Thanks for the info. Guess I have to wait for some team of physicists to tackle that one :) While reading Ethan's post I started thinking about the solar system, and was just curious about how many stars are need to make enough heavier elements to i.e. get a solar system. Is 1 solar mass enough or are i.e. 20 solar masses needed or 2000? That's why I asked.
p.s.
further down the road I guess one could then use that info and backtrack our own galaxy. If you knew how much of what there is now in our solar neighborhood, you could say how it looked before. Sort of like star archeology. To trace from where the elements that make planets came from. Not in general, but specifically. Like.. we need 200 solar masses in the region of 40 light years some 10 billion years ago in order to make what we have now :)
linked to my blog and some photos borrowed (using my bandwidth). Great post.
Wow,
I've read enough of your posts to know that this was probably a typo, but you said, "the universe can't be any older than any star you see in it". Obviously you meant the opposite, right? The universe can't be any younger than any star you see in it. Your statement would imply that all stars currently observable must have been present since the beginning of the universe, which is of course not true.
Oopse :-)
"Thanks for the info. Guess I have to wait for some team of physicists to tackle that one"
Well, it's been tackled, but from the other direction.
It's a bit like using a steel ruler to measure the wavelength of light. You can do it, but why the heck do it in such an ass-backward way.
So the universe has been simulated with a physically plausible spread of star sizes and the result is wound on through generation after generation.
The relative abundance of the elements isn't the only thing retrieved.
What you may want to do is an ensemble hindcast to see how convergent it is. If it's pretty finely covergent, then you could use the measured abundance on this universe and check off the mean ensemble run universe's relative abundancies and then tick it off as "the most likely".
Ethan may know someone who does this sort of simulation and could pass on the info for the models run or what result they got.
The numbers in physics are so extravagant, so large, so small, so different (complex variables etc.) and physics itself so wonderfully crazy, Alice-in-Wonderland, so entirely counterintuitive. I'd sure like to wade thru the math necessary to understand it but it'd be difficult.