"I see a lot of new faces. But, you know the old saying, 'out with the old, in with the nucleus.'" -The Simpsons
Looking around the Universe today, there's no doubt that there's plenty of hydrogen and helium around; after all, it's the nuclear fusion of hydrogen into helium that powers the vast majority of stars illuminating the entire cosmos!
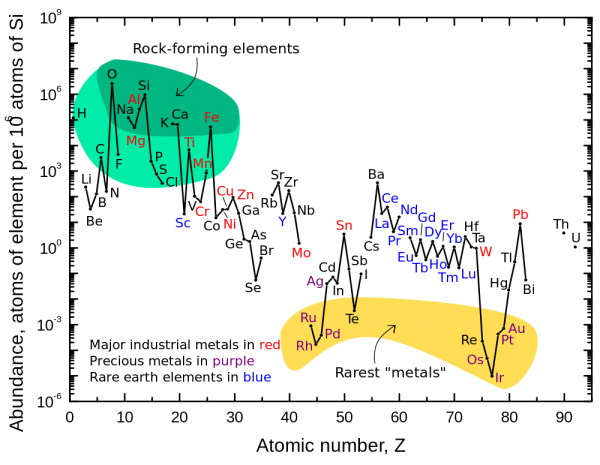
But here on Earth, hydrogen and helium are only a small part of the world we inhabit. By mass, hydrogen and helium combined make up far less than 1% of the Earth, and even if we restrict ourselves to the Earth's crust, it's still just a tiny percentage compared to the other, heavier elements.
Practically all of these heavy elements were formed in generations of stars: stars that lived, burned their fuel into heavier elements, died and shed their heavy, enriched elements back into the cosmos, and were incorporated into the next generations of stars and -- when the heavier elements became abundant enough -- rocky planets.
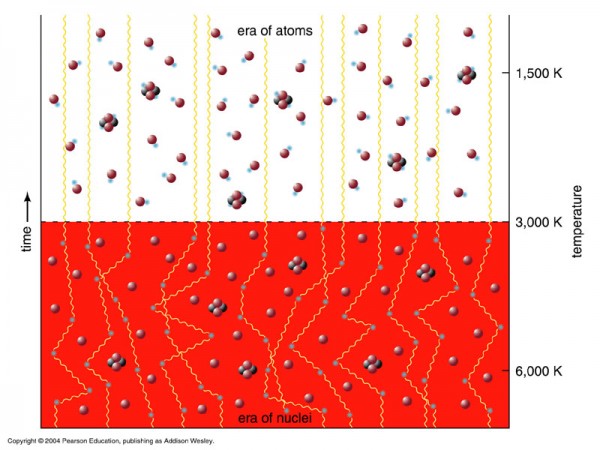
But the Universe didn't start off with these heavier elements at all. In fact, if you'll remember what the Big Bang says, the Universe is expanding (and cooling) now, meaning that all the matter in it was closer together -- and the radiation in it was hotter -- in the past. If you go back to a sufficiently early time, you'll find that the density was high enough and the temperature was hot enough that you couldn't even form neutral atoms without them immediately being blasted apart! When the Universe cooled through that phase, that's when neutral atoms formed for the first time, and where the cosmic microwave background comes from.
At that time, the Universe was made out of about 92% hydrogen atoms and 8% helium atoms by number (or about 75-76% hydrogen and 24-25% helium by mass), with trace amounts of lithium and beryllium, but not much else. But you might wonder how it got to have exactly that ratio? After all, it didn't have to be that way; if the Universe was hot and dense enough to undergo nuclear fusion early on, why did it only fuse atoms up to helium, and why didn't more of the Universe become helium than it did?
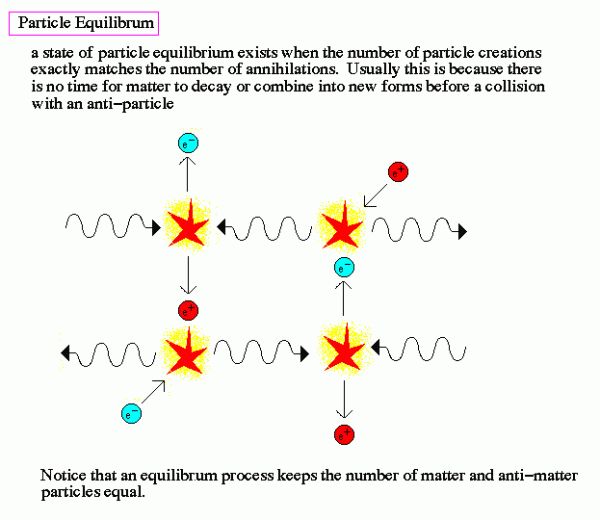
To find the answer, we need to go way back in time. Not just to the first few hundred thousand years of the Universe, when it was making the first atoms, nor even to the first years, days, or hours. No, we need to go back to when the temperatures were so high, when the Universe was so hot, that not only could atomic nuclei not form (for they'd be immediately be blasted apart), but to a time when the Universe was so hot that the Universe was filled with nearly equal amount of matter-and-antimatter, when it was just a fraction of a second old!
It was once so hot that the Universe was filled with nearly equal amount of matter and antimatter: protons and antiprotons, neutrons and antineutrons, electrons and positrons, neutrinos and antineutrinos, and of course photons (which are their own antiparticle), among others. (They're not exactly equal; see here for more on that.) When the Universe is hot -- and by hot, I mean above the temperature needed to spontaneously create a matter/antimatter pair from two typical photons -- you get huge amounts of that form of matter and antimatter. They get spontaneously created from photons just as quickly as they find one another and annihilate back into photons. But as the Universe cools, those matter/antimatter pairs begin to annihilate faster, and it becomes more difficult to find photons energetic enough to make them. Eventually, it cools enough that all the exotic particles go away, and all the antiprotons and antineutrons annihilate with protons and neutrons, leaving only a small asymmetry of matter (in the form of protons and neutrons) over antimatter, bathed in a sea of radiation.
At this point, when the Universe is a fraction of a second old, there are roughly equal amounts of protons and neutrons: about a 50/50 split. These protons and neutrons will eventually become the atoms in our Universe, but they've got a lot to go through first. On the other hand, electrons (and positrons) are much lighter, so they still exist in huge numbers (and at great energies) for a while longer.
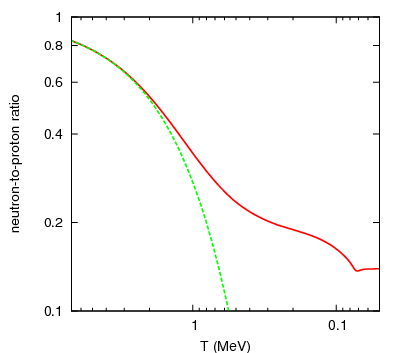
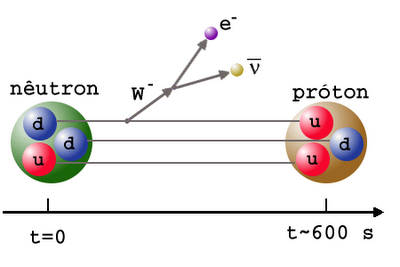
It's still hot enough that protons and neutrons can convert into one another very easily: a proton can combine with an electron to make a neutron and (an electron) neutrino, while a neutron can combine with (an electron) neutrino to make a proton and an electron. While there aren't that many protons and neutrons in the Universe at this time, electrons and neutrinos outnumber them by around a billion-to-one. This is why, early on, there's about a 50/50 split of protons and neutrons.
Neutrons, as you'll remember, are slightly heavier than protons: by about 0.2%. As the Universe cools (and the excess positrons annihilate away), it becomes rarer and rarer to find a proton-electron pair with enough energy to create a neutron, while it's still relatively easy for a neutron-neutrino pair to create a proton-electron pair. This converts a substantial fraction of neutrons into protons during the first one-to-three seconds of the Universe. By time these interactions have become insignificant, the proton-to-neutron ratio has changed from about 50/50 to 85/15!
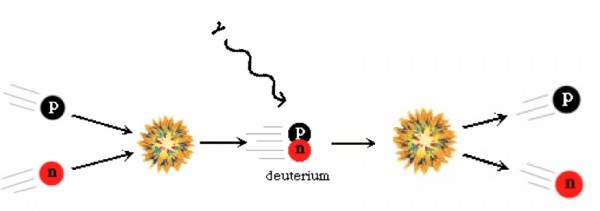
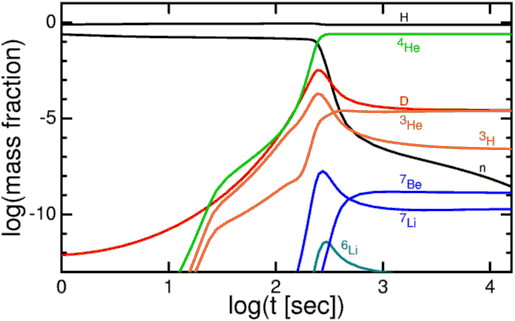
Now, these protons and neutrons are abundant, hot, and dense enough that they can fuse together into heavier elements, and believe me, they'd love to. But photons -- particles of radiation -- outnumber protons-and-neutrons by more than a billion to one, so for minutes of the Universe expanding and cooling, it's still energetic enough that every time a proton and neutron fuse together to form deuterium, the first stepping-stone in nuclear fusion, a high-enough energy photon immediately comes along and blasts them apart! This is known as the deuterium bottleneck, as deuterium is relatively fragile, and its fragility prevents further nuclear reactions from occurring.
In the meantime, while the minutes tick by, something else is going on. A free proton is stable, so nothing happens to them, but a free neutron is unstable; it will decay into a proton, electron, and an (electron) antineutrino with a half-life of about ten minutes. By time the Universe has cooled enough that the created deuterium wouldn't be immediately be blasted back apart, more than three minutes have gone by, further changing the 85%-proton/15%-neutron split to nearly 88% protons and just a hair over 12% neutrons.
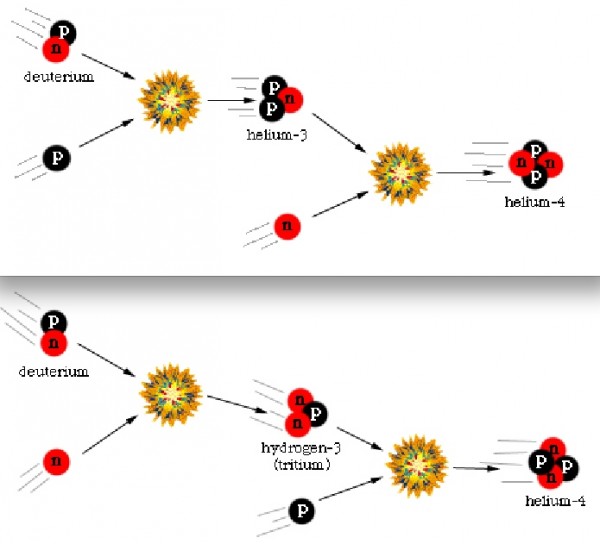
Finally, with deuterium forming, nuclear fusion can proceed, and it proceeds extremely rapidly! Through a couple of different fusion chains, the Universe is still hot and dense enough that pretty much every neutron around wind up combining with one other neutron and two protons to form helium-4, an isotope of helium that's much more energetically stable than deuterium, tritium, or helium-3!
By time this happens, though, the Universe is nearly four minutes old, and is far too diffuse and cold to undergo the next major step of fusion that happens in stars, which is to fuse three helium-4 atoms into carbon-12; that process will have to wait tens of millions of years until the Universe's first stars form!
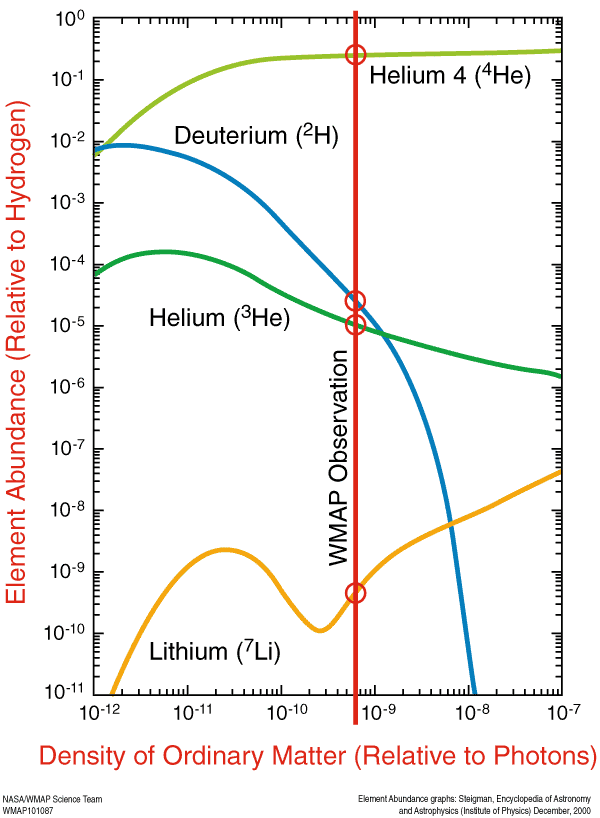
But these nuclei are stable, and there will also be a trace amount of helium-3 (which tritium will also decay into, eventually), deuterium (hydrogen-2), and very small amounts of lithium (and probably even smaller amounts of beryllium) formed by very rare fusion reactions.
But the overwhelming majority of neutrons -- 99.9%+ of them -- wind up locked up in helium-4 nuclei. If the matter in the Universe contained just a hair over 12% neutrons and just a hair under 88% protons just prior to nucleosynthesis (the fusion into heavier elements), that means that all of those neutrons and and equal amount (just over 12% of the Universe) of protons winds up becoming helium-4: a total of 24-to-25% of the mass, leaving 75-to-76% of the Universe as protons, or hydrogen nuclei.
So that's why, by mass, we say 75-76% was hydrogen and 24-25% was helium. But each helium nucleus is around four times the mass of a hydrogen nucleus, which means that, by number of atoms, the Universe is around 92% hydrogen and 8% helium.
This primordial, unprocessed material has actually been detected observationally, and is one of the three cornerstones of the Big Bang, along with Hubble expansion and the cosmic microwave background. And that's where all the elements in the Universe started from! Everything you are, everything you know, and every material object you've ever interacted with came from this primordial sea of protons and neutrons, and was once a mere collections of hydrogen and helium atoms. And then the Universe happened...
and here it all is! And that's where -- if you go way, way back -- all the atoms came from.
- Log in to post comments



Yet another nice article. I like the way the illustrations break up the text and make it easy reading.
Something that doesn't feature in the standard description is that you can "melt" hadrons in a "quark-gluon plasma", see http://physicsworld.com/cws/article/print/2009/sep/01/of-gluons-atoms-a…. I think this might turn out to be important for baryon and lepton asymmetry myself, wherein positrons are actually more like protons than electrons are. That's another one for another day!
Rad! I'm always curious as to why and how there was "small amounts of lithium" in the very early stages on the universe, but I think I grok the random fusion chain now. If it is something else, please tell me.
Also, Ethan, relevant to this post: a friend and very talented musician/artist Kim Boekbinder AKA The Impossible Girl just released a new space-themed album, called "The Sky is Calling"!!
Youtube for the first music video from the album, for the song "Stellar Alchemist" here: http://www.youtube.com/watch?v=ENJKo5jqjUw&feature=youtu.be
You and readers will appreciate it I'm sure.
Excuse me for thinking, but what if time is more than a marker of passage, what if it is the fundamental particle, intertwined with space, what if it is time that exploded creating the big bang? Excuse me for thinking.
You will need to actually specify what the hell you're on about, Tony. Because what you wrote just there was meaningless garbage.
What if you reversed the osmosis of the dilithium crystals and injected red matter to cause a time skip in a bagel?
xcuse my garbage.
No.
Wow, what is your definition of time. Why does time slow down near a large mass such as a black hole, why does time stop at the speed of light, the twins scenario. Why should time vary at all. It does, but why? What is time? Explain it to me. Why is the arrow of time what it is? I know you must know, not just speculation.
wow, If time stopped so would the universe instantly disappear. The Universe and time began at the same instant, so, maybe time is a something, maybe it's the power behind inflation. the basic wave function of all functions, that permeates all of space and matter. When people have experience NDE they say that time essentially stopped they couldn't tell if they had been gone for a second or a much longer time, so if this is true than time is a property of this universe alone, barring other universes that may exist, and this is also essentially speculation by Physicists, though I believe this may well be. So, science begins with imagination, and a desire to know.
they are experiencing a chemical imbalance in the brain, nothing more - certainly nothing indicative of any deep notions about the universe.
Everyone experiences an NDE.
It's called "life".
"If time stopped so would the universe instantly disappear."
If time stopped, how is there an instant for something to disappear in?
The ancient Greeks had this problem, Zeno's paradox it is called.
Most people know of it but know that it is a fallacious reasoning problem, even if they don't know why.
You're still at the "Well, how DOES the arrow catch up to the tortoise?!?!".
"Why does time slow down near a large mass such as a black hole"
It doesn't. For the thing in that situation, time moves just as fast as it always did.
Maybe Ethan can explain this at some time.
wow, time does dilate relative to other objects who are not near the massive object.
Tony Rotz: You're confusing the physics notion of subjective time -- the relative timing of events may differ for different observers -- with the psychological notion of time perception. This is what your brain does, creating a cohesive narrative and giving you a sense of how much time passes. And just like everything else our brain does on our behalf, this involves a lot of fudging, filling in blanks, and outright lies.
There have been experiments done on NDEs and the perception that time slows down. While people in the experiments claimed that time slowed down and they could see everything in complete detail, their measured ability to recall those details correctly was no better than normal. Events that occurred too quickly to be perceived normally were also not perceived during the experiment.
In short, it truly is only the *perception* of time that changes. Not actual time, and not any other form of perception. The brain just fills in a bunch of details because it decided that event took much longer than it really did, and therefore you should have been able to see a lot more than you normally would. So what you get is a seemingly self-consistent picture (cus that's what your brain does) that falls apart when put to the test.
Gleaning physical insight from this psychological quirk is like believing that the moving-image optical illusions Ethan posted a week or two back means the images are REALLY moving.
Oh and by the way this is expected -- as Wow hinted, the changes in time are only visible *relative* to something else. No matter how "fast" or "slow" time is going relative to somewhere else, you will always observe time locally to be going at the same rate (think about it).
The chance of you comprehending a response is zero, Tony.
wow, why don't you use your real name, if your going to insult someone instead of hiding behind a wow. What's wrong with you? This blog shouldn't be used for insults, grow up. Your hatred is showing.
I'll stay off this blog rather than cause your hatred to grow. good bye.
Uh, what the hell? No hate, dude, just completely sick and tired of meaningless drivel.
Tony, why do I have to use my real name? It is no more me than my pseudonym. Giving it to you will give you no more info than a fake one and you can find nothing else out about me from it.
If someone measures 50 grams 20 times, they still get 1Kg even if the mass of a standard kilogram changes. If the yardstick is half as long, but everything around it is also half as long... This is why it's called relativity. ;)
It's a scaling problem. Does that explain it? This is related to the term 'dimensionless constants'. Like if the universe octupled in volume but the reactions as related to the size of an object stayed the same, we would not be able to tell that everything became twice as far away. For extra fun, read up on something called 'doubly special relativity' and all the issues involved in a maximum frequency/velocity/density and Planck units. It's not like we can actually test to see what happens when a photon has a wavelength approaching the Planck length unit. It would take more energy than we can even imagine being applied by human technology.
@Helyx, great lyrics and videoclip indeed. I already bookmarked it :)
Anyway, I made a Dutch translation of this article:
http://www.astroblogs.nl/2013/07/15/nucleosynthese-en-de-oerknal/
love the pics on this web page excuse me for being a geek but its awesome.
SCIANCE RULES!!!!!!!!!!!!!!!
everything is fine but you unlike every other major site in the world has missed the most important point, how did protons, neutrons, electrons, quarks came into existence FROM NOTHING? the big bang was just a massive explosion that only had light, where did all these constituents come from?
There's a thread for that
http://scienceblogs.com/startswithabang/2011/02/02/can-you-get-somethin…
odd that you complained well after that thread was started about how no site had such a thread...
So the question is: if a site had such a conversation, would you ever have bothered to read it?
I think the answer is an emphatic "no".
Every going day, the number of thinking brains is increasing; this is pushing us (humans) of understanding more and more. Now a days, more than 6 billion thinking brains making a strong power to explore whether by NASA rockets or even by seating, drinking a cup of tea, thinking scientifically in it.
Human found that it’s a rounding ball, not a flat earth, visited the moon, well, voyjar has did a good job far away of home, in the same time scientists are working home making diamond in the labs,
The point I'm trying to clear is that everyone has the rights to think and to share, not has the rights only but HAS TO THINK AND SHARE, so please respect everyone's point.
Wish you all the best, hamed from Saudi hamedalshahrani@yahoo.com
Wow...Wow, it's people like you that slow down the progress of humanity. Just reading the comments above between you and Tony was the most frustrating aspect of my day. What do you get out of being so arrogantly pompous? I agree, what Tony was saying may not align with what science currently tells us, but it's an interesting thought and you attack it as if you know all the answers to the universe. Who knows what we might discover in another 100 years - the way we understand and interpret the universe is constantly changing as we discover more incredible things. I'm not saying your argument was invalid or incorrect, you seem to be very intelligent; I'm just saying that it makes you look incredibly weak by instantly shutting down a fellow thinker who is just trying to have an intellectual "outside of the box" conversation.
I'm sure you will respond with some sort of arrogant remark - well so be it - it was just hard to read this thread without trying to teach you a little bit of decency.
John Schmit, that is well said. I had the exact same reaction from reading this thread.
If the universe is expanding why the earth's distance to the sun is still the same? If the universe started from nothing or from tiny particle how did it grow?
@mario de vera #35: Because (1) the Earth and Sun are bound together gravitationally, and (2) the expansion rate is so tiny as to be unmeasurable on Solar System scales!
The expansion rate, today, is 67 km/s per megaparsec. That is, two galaxies one megaparsec apart would be separating at 67 km/s. Two galaxies ten megaparsecs apart would be separating at 670 km/s. And so on.
The Earth and Sun are 150 million km apart (on average). That's five TRILLIONTHS of a megaparsec (4.86e-12 Mpc). So the cosmic expansion is trying to separate the Earth and Sun at a rate of 0.32 billionths of a km/s (0.32 microns/second). The Sun's gravity is such that escape velocity at the position of Earth's orbit is 30 km/s, or ten billion times faster than the cosmic expansion rate. To paraphrase an old song, "Gravity, gravity will keep us together..."
It isn't just Wow's comments to Tony, but also to Big Sooka, that make me agree with IMHO and John's sentiments re pompous arrogance. The amusing thing is that Wow is hiding behind the theories we accept today that have been cleverly woven together by others, not by Wow. Judging from the attitude to others, I wouldn't even suggest Wow is intelligent. All that has come from Wow's input here is regurgitation gleaned by memorising detail. Not even one attempt at an imaginative original thought. Like what initiates particle/antiparticle creation? Why is the speed of light limited ? Is the cosmological principle valid ? Hide behind the shoulders of giants then, Wow.
@ian b #37: Wow has an excruciatingly harsh response to non-scientific comments. However, his responses are quite on target technically. If you are having a _scientific_ discussion, then you are best served by sticking with known science.
If you want to have an idly speculative discussion, then by all means wander off into non-scientific speculations.
Smart people, Stupid questions... (Not to offend anyone, we all have stupid questions.)
If the universe started from a single point, and that it is expanding, how is it possible for two galaxies to collide (eg: milky way and andromeda collision)?
Because space is moving, but the things are moving in that space.
Hi
You write:"By time this happens, though, the Universe is nearly four minutes old, and is far too diffuse and cold to undergo the next major step of fusion that happens in stars, which is to fuse three helium-4 atoms into carbon-12; that process will have to wait tens of millions of years until the Universe’s first stars form!"
I present my hypothesis: carbon production :)
7Li(p,y)8Be*(a,y)12C*
Link:https://scontent-vie1-1.xx.fbcdn.net/hphotos-xta1/v/t1.0-9/12243382_108…
Lithium-proton reaction channels:
Link:https://scontent-vie1-1.xx.fbcdn.net/hphotos-xpa1/v/t1.0-9/12310578_109…
This hypothesis explains: 2H, 6Li, 7Be, n, Nucleosynthesis.
Regards nyemi
Write it up and present to a peer reviewed paper where your ideas will be discussed and viewed by those able to critique it without them worrying about wasting time on a crank site or vanity blog. When it gets past peer review, let us know.
Ok :).
Thank you, your reply.
Physics: "that which is not forbidden is mandatory".
Regards nyemi
Why on earth do you claim that? Obviously, the answers you got did not manage to make any impression on your knowledge.
That which is not forbidden is possible.
Regards, the genus homo sapiens sapiens.
Link:http://arxiv.org/abs/1602.07298
Yeah, still says fuck all about how you come to the assertion "that which is not forbidden is mandatory”.
Quite why you think it was relevant is probably from the same lack of intelligence as caused the claim in the first place.
Perhaps someone here can help me, because it is an extremely arduous task to find the answer on Google.
Stars fuse hydrogen into etc. etc. and dies once most of its hydrogen is depleted. Some stars supernova, thereby creating a nebula. More stars are born from this and the whole process starts over.
My question is, since most of the hydrogen was fused in the first star, where does all the hydrogen come from to fuel the next generation of stars?
Any help would be appreciated.
a supernova does not necessarily create new stars unless it is in a giant nebula itself, like the eagle nebula, in which case it is just adding to the elements in the cloud, a lone star like ours that supernovas just blows its cloud into space, like the crab nebula
@Andre #51: What you described for a star's lifetime applies fairly well to the Sun and other light-weight stars. The heaviest stars, the ones which produce supernovae and heavy elements, don't burn "most of [their] hydrogen." Rather, what we believe happens is that in their dense cores the hydrogen all burns to helium, then to carbon and oxygen, then to silicon, and so on up to iron. Since the temperature and pressure both get lower as you move out from the core, what we expect is that there will be "layers" (probably somewhat mixed) where different kinds of fusion are occurring. The outer envelope of the star (with a significant part of the star's mass) is likely to still be mostly hydrogen by the time the star explodes.
At the same time, when a supernova does happen and spreads heavy elements out into the nearby environment, that material is going to be mixed together with the mostly hydrogen and helium which is already there.
"75-76% of the Universe is hydrogen, 24-25% is helium." 101%?! What about the other elements?
Usually, this is explained as this Universe is ~74% hydrogen, ~24% helium, and ~2% all the other elements.
You've modified the text. It would be best to go back to the beginning and note the words "at that time."