“Art has a way of confronting us, of reminding us, of engaging us, in what it means to be human, and what it means to be human is to be flawed, is to be contradictory, is to be often weak, and yet despite all of these what we would consider drawbacks, that we’re also quite beautiful. Spin is the opposite.” -Junot Diaz
"Two identical fermions cannot occupy the same state," says the Pauli Exclusion Principle, one of the most fundamental laws of quantum physics that governs our Universe. But if that's truly the case, then how can two hydrogen atoms bind together?
This deceptively simple question actually has a fascinating answer, and as it turns out, it holds the key to not only how they can bind together to form molecular hydrogen, but also to why you can bind no more than two hydrogens together!
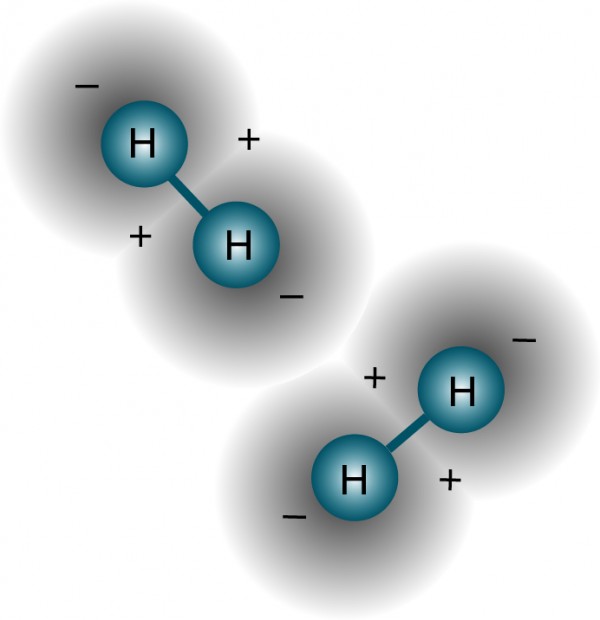 Image credit: CERN, 2001, via http://www.physicsmasterclasses.org/exercises/keyhole/it/theory/main-5….
Image credit: CERN, 2001, via http://www.physicsmasterclasses.org/exercises/keyhole/it/theory/main-5….
Go and read the whole thing here.
- Log in to post comments






@Ethan: Very nice! One editorial comment, and one question:
In your last paragraph, I think you wanted to write "hydrogen molecule," not "hydrogen atom."
The very nice set of 2D plots from New Scientist -- do you happen to have a reference for the original paper? The figure itself just says (lower right margin) "SOURCE: PHYSICAL REVIEW LETTERS." Well, duh :-)
Michael: is this it?
IMHO the thing to remember is that "electrons exist as standing waves" in atomic orbitals. And they don't suddenly start existing as something else when they get ejected. That's why you can diffract electrons.
I like your spin explanation very much, but I want to ask the following: Would it be possible to collect a lot of hydrogen atoms exclusively with aligned spins (not bonding) to obtain atomic hydrogen gas? And what properties would it then have?
The diagrams are lovely and fascinating.
In this exposition devoted to the behavior of electrons in hydrogen, I thought you could have included some discussion on the other type of hydrogen atom known to exist in the photospheres of stars like the sun : the negative hydrogen ion in which both electrons stick to the same atom.
Its discovery in the sun is a fascinating chapter!
Thanks Bill and Ethan.
If H2 is formed by symmetric and anti-sysmmetric H atoms does this mean there are 2 species of H2?
Charles Herring,
I think you may have misunderstood. A bond can only be formed if the electrons are in the spatially symmetric, spin anti-aligned state. That configuration results in a bonding orbital. The spatially anti-symmetric, spin aligned state produces an orbital in which there is no electron density overlap between the two nuclei, ie. an anti-bonding orbital.
Basically, the spatially antisymmetric configuration is equivalent to two free hydrogen atoms and is less energetically stable than the bonding configuration. That is why we see diatomic hydrogen rather than free hydrogen atoms.