Thermodynamics
I recently spent a week in Lithuania visiting biophysical laboratories and giving a couple of seminars. My host was Daumantas Matulis of the Institute of Biotechnology at Vilnius University, where they have an EU grant that includes funds for bringing in visiting scientists from other countries (thank you EU!). Although my sampling of the science in Lithuania is quite limited – the labs that I visited exhibited some interesting similarities. Physically, the labs at the Institute of Biotechnology in Vilnius and in the Institute of Cardiology in Kaunas consist of large collections of small…
Okay, after a long, long gap (on the blogosphere timescale) and/or almost zero elapsed time (by scientific literature standards), we're going to attempt to wrap up this mini-series on heat capacity effects in biology. Parts 1 and 2 are here and here, respectively.
So: How do you know if your reaction has a heat capacity change? Actually, it's easy: collect data as a function of temperature and make a van't Hoff plot (ln 1/Keq versus ln 1/temperture) or a Gibbs-Helmholtz plot (ÎG versus temperature) - if either plot is not-linear your reaction has a ÎCp.
This figure shows Gibbs-Helmholtz…
The laws of thermodynamics are empirical laws - they were not derived from some first principles of the universe: they were derived by doing thousands and thousands of experiments, and then coming up with some relationships that could quantitatively explain all those experiments.
In biological thermodynamics, we are at the beginnings of trying to define a similar set of "laws of biothermodynamics" - in this case we want relationships that connect thermodynamic quantities (ÎG, ÎH, ÎS, ÎCp) to functional or structural information about the biomolecules themselves. Nobody has anything that…
Scienceblogs is promoting the writing of "Science 101" general topic posts all through the "back to school" month of September. So, here is the first in a multi-part series on Heat Capacity in Biology:
Heat Capacity in Biology 101: What is it?
Heat capacity is basically a proportionality constant. For any substance, the heat capacity tells you how much the temperature of the substance will change when you add a specific amount of heat.
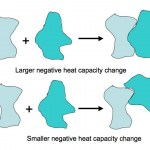
Here is an absolutely beautiful schematic illustration of the difference between a small heat capacity and a large heat capacity (from a website on the…
I got forwarded a physics question last night asking about the connection between wind and temperature, which I'll paraphrase as:
Temperature is related to the motion of the atoms and molecules making a substance up, with faster motion corresponding to higher temperature. So why does it feel warmer when the air is still and why does wind make you feel cold?
This is a moderately common point of confusion, so while I responded to the question in email, I'll also appropriate it for a post topic. So, why doesn't "windy" equal "hot," given that wind consists of moving air molecules?
The full…
Regular readers will recall a recent post pointing to Dr Roy Spencer being cannabalized cannibalized over his stubborn insistence that the Greenhouse Effect does not violate the laws of thermodynamics.
Well, he seems to be a glutton for punishment as he is taking another crack at it.
This time, I am only pointing it out because he has taken a high tech experimental approach to observe the actual atmospheric back radiation and an interesting post results (hi tech compared to his last hotplate device!).
I just skimmed the comments, like last time, and while the die-hards are still hard to…
Back at the start of the summer, I asked a question about automotive thermodynamics: On a hot day, is it better to open your car windows a crack when making a short stop, or leave them closed? For a long term-- say, leaving your car parked outside all day-- I hope everyone will agree that leaving the windows slightly open is the better call, but the answer isn't as clear for a short stop. There might well be some time during which the open-window car heats up faster as warm air from outside gets in, while the closed-window car holds in the air-conditioned goodness longer.
It occurred to me…
Over at Uncertain Principles, Chad frets about committing physics heresy via a reading of Goldilocks and the Three Bears to his young offspring.
The story may convey a useful moral message, but it's way off base on the physics.
After all, the Papa Bear, being the biggest, presumably has the largest bowl of porridge. Here, the story fits what we know about thermodynamics, as the largest bowl should take the longest time to cool, and thus should be the hottest at any time before the porridge bowls reach thermal equilibrium with their environment.
The description provided of the other two bowls…
There are some really cool questions out there. Questions that do not require a lot to ask and do not require a lot to answer. Here is one such question (I can't remember where I first found this question or something like it):
Suppose you put take two identical cans of soda out of the fridge and place them on the floor in the middle of a room. One can you leave alone and one can you cover with a wool blanket. After an hour, you come back and check on the two cans of soda. Which will be warmer?
First, why do I like this question? Mainly because everyone can give an answer. If asked…
This is just one of dozens of responses to common climate change denial arguments, which can all be found at How to Talk to a Climate Sceptic.
Objection:
The so called "Greenhouse Effect" which is the underpinning of the entire theory of anthropogenic global warming claims that greenhouse gases in the upper atmosphere absorb outgoing long wave radiation from the surface and reradiate it back, thereby warming the climate. But the upper atmosphere is colder than the lower atmosphere and the surface and the second law of thermodynamics clearly requires that heat flow from warmer areas of a…
tags: ice-nine, supercooled water, physics, chemistry, thermodynamics, streaming video
This is a fun little experiment with water that was supercooled to -21C (-6F). The supercooled water is poured into a bowl. It pours out as a liquid and turns to slush, forming ropelike peaks [1:07].
What are the physics behind how water can be supercooled without freezing into a solid? It happens like this; when water is supercooled but remains undisturbed, it does not freeze into a solid unless there is an impurity present (a rough surface, for example) for the cold water to crystallize around (a "…