Element: Lithium (Li)
Atomic Number: 3
Mass: Two stable isotopes, masses 6 and 7 amu
Laser cooling wavelength: 671 nm
Doppler cooling limit: 140 μK.
Chemical classification: Alkali metal, column I in the periodic table. Yet another greyish metal. We're almost done with alkalis, I promise. Less reactive than any of the others, so the explosions in water aren't very impressive.
Other properties of interest: Lithium-7 is a boson, but has a negative scattering length, meaning that BEC's of lithium-7 tend to implode unless you modify the collisional properties. Lithium-6 is a fermion, and much…
Element: Francium (Fr)
Atomic Number: 87
Mass: Numerous isotopes ranging in mass from 199 amu to 232 amu, none of them stable. The only ones laser cooled are the five between 208 amu and 212 amu, plus the one at 221 amu.
Laser cooling wavelength: 718 nm
Doppler cooling limit: 182 μK.
Chemical classification: Alkali metal, column I in the periodic table. The heaviest known alkali, it's presumably metallic in appearance, if you could ever get enough of it together to look at.
Other properties of interest: Francium has no stable isotopes, and the longest lifetime of any of its isotopes is around…
The list of editions of my books in character sets I can't read just got bigger: I got author copies of the Thai edition of How to Teach Physics to Your Dog. The cover is the "featured image" above (and I'll copy it below for those on RSS), and I love the goofy tongue on the cartoon German Shepherd. The bunny-squirrel-steak atom is also pretty sweet.
Of course, I can't assess the translation at all-- honestly, I don't even know which was the script runs. The pages are all right-justified, so I'm guessing it reads right-to-left, but I haven't the foggiest idea whether the translation does it…
In one of those Information Supercollider moments, two very different articles crossed in my social media feeds, and suddenly seemed to be related. The first was this New York Post piece by a college essay consultant:
Finally, after 15 or so years of parents managing every variable, there comes the time when a student is expected to do something all by herself: fill out the actual application. Write an essay in her own voice.
By this point, our coddled child has no faith in her own words at all. Her own ideas and feelings, like a language she has not practiced, have fallen away.
Her parents…
Element: Strontium (Sr)
Atomic Number: 38
Mass: Four stable isotopes, ranging from 84 to 88 amu
Laser cooling wavelength: Two different transitions are used in the laser cooling of strontium: a blue line at 461 nm that's an ordinary sort of transition, and an exceptionally narrow "intercombination" line at 689 nm.
Doppler cooling limit: 770 μK for the blue transition, below a microkelvin for the red. The Doppler limit for the red line turns out not to be all that relevant, as other factors significantly alter the cooling process.
Chemical classification: Alkaline earth, column II of the…
Early last year, we began marking SteelyKid's height off on a door frame in the library. She occasionally demands a re-measurement, and Saturday was one of those days. Which made me notice that we now have a substantial number of heights recorded, and you know what that means: it's time for a graph.
The "featured image" above shows the results, and I've stuck in a zero-day point using her length at birth. The solid line in the graph is a linear fit to the data, because nothing could possibly be wrong with that. According to the fit, we can project that she'll reach a height of 3.0 meters a…
Adam Frank has an op-ed at the New York Times that tells a very familiar story: science is on the decline, and we're living in an "Age of Denial".
IN 1982, polls showed that 44 percent of Americans believed God had created human beings in their present form. Thirty years later, the fraction of the population who are creationists is 46 percent.
In 1989, when “climate change” had just entered the public lexicon, 63 percent of Americans understood it was a problem. Almost 25 years later, that proportion is actually a bit lower, at 58 percent.
The timeline of these polls defines my career in…
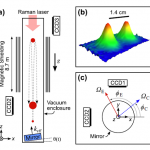
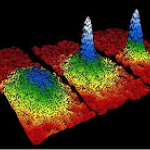
When I wrote up the giant interferometer experiment at Stanford, I noted that they've managed to create a situation where the wavefunction of the atoms passing through their interferometer contains two peaks separated by almost a centimeter and a half. This isn't two clouds of atoms each definitely in a particular position, mind, this is a wavefunction representing a bunch of atoms that are each partly in two places at the same time , separated by 1.4 centimeters.
I emailed Mark a link to the post, and in his reply he said that they've increased that to about 4cm (which is just a matter of…
Element: Xenon (Xe)
Atomic Number: 54
Mass: nine "stable" isotopes, masses from 124 to 136 amu. Xenon-136 is technically radioactive, but with a half-life of a hundred billion billion years, so, you know, it's pretty much stable.
Laser cooling wavelength: 882 nm
Doppler cooling limit: 120 μK
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with anything, so poses much less danger to scientists than any of the alkalis, though it has, at times, been used as an anesthetic, and Will Happer's group at Princeton has a funny story about a student's…
After a couple of very productive days where I closed my Twitter tab because it was too freakin' annoying to read, I checked in briefly Wednesday morning, and found Rhett Allain and Frank Noschese discussing this Veritasium bullet-in-block experiment:
Tom at Swans On Tea offers some analysis, and Rhett offers a video response doing out some of the math:
I basically agree with their explanations, and that should have been that, except that in his discussion Rhett mentioned that the bullet probably doesn't go as far into the spinning block, which prompted Frank to ask whether you could…
Element: Helium (He)
Atomic Number: 2
Mass: two stable isotopes, 3 and 4 amu.
Laser cooling wavelength: 1083 nm
Doppler cooling limit: 38 μK
(It should be noted, though, that despite the low temperature, laser-cooled helium has a relatively high velocity-- that Doppler limit corresponds to an average velocity that's just about the same as for sodium at 240 μK. This is because temperature is a measure of kinetic energy, and helium is much, much lighter than any of the other laser-cooled elements.)
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with…
I'm writing a bit for the book-in-progress about neutrinos-- prompted by a forthcoming book by Ray Jaywardhana that I was sent for review-- and in looking for material, I ran across a great quote from Arthur Stanley Eddington, the British astronomer and science popularizer best known for his eclipse observations that confirmed the bending of light by gravity. Eddington was no fan of neutrinos, but in a set of lectures about philosophy of science, later published as a book, he wrote that he wouldn't bet against them:
My old-fashioned kind of disbelief in neutrinos is scarcely enough. Dare I…
A little over a year ago, I visited Mark Kasevich's labs at Stanford, and wrote up a paper proposing to use a 10-m atom interferometer to test general relativity. Now, that sounds crazy, but I saw the actual tower when I visited, so it wasn't complete nonsense. And this week, they have a new paper with experimental results, that's free to read via this Physics Focus article. Which might seem to make me blogging it redundant, but I think it's cool enough that I can't resist.
OK, dude, "Multiaxis Inertial Sensing with Long-Time Point Source Atom Interferometry" is not the sexiest title in the…
Element: Rubidium (Rb)
Atomic Number: 37
Mass: two "stable" isotopes, 85 and 87 amu (rubidium-87 is technically radioactive, but it's half-life is 48 billion years, so it might as well be stable for atomic physics purposes.
Laser cooling wavelength: 780 nm
Doppler cooling limit: 140 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it’s a greyish metal at room temperature. Like the other alkalis, it’s highly reactive, and bursts into flame on contact with water, even more so than sodium (in general, the alkalis get more violently reactive…
Element: Sodium (Na)
Atomic Number: 11
Mass: one stable isotope, 23 amu
Laser cooling wavelength: 589 nm
Doppler cooling limit: 240 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it's a greyish metal at room temperature. Like the other alkalis, it's highly reactive, and bursts into flame on contact with water. For this reason, all physicists working with sodium have True Lab Stories about accidentally blowing stuff up with it.
Other properties of interest: Scattering length of around 80 a0; Feshbach resonance at around 900 G.
History:…
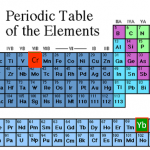
At the tail end of the cold-atom toolbox series, I joked about doing a "trading card" version shortening the posts to a more web-friendly length. In idly thinking about this, though, it occurred to me that if one were going to have cold-atom trading cards, it might make more sense to have them for the atoms, rather than the techniques. And having just devoted many thousands of words to technique, I don't really feel like trying to cut those down more, but atoms...
The "featured image" up top is a slide from my laser cooling lectures for our first-year seminar class. Elements outlined in red…
The "featured image" above shows SteelyKid and The Pip checking out a couple of books at The Open Door during our weekly trip to the Schenectady Greenmarket. As cute as this is, though, the image can't do justice to the full scenario.
We were in the toy section looking for a birthday present for SteelyKid's BFF, and after she found a couple of things, she announced her intention to look at books in the cheap paperback racks right next to the toys. The Pip, for his part, waddled off to poke around the store looking for books featuring Elmo, or things that look like Elmo, or just odd bits of…
SteelyKid: I din't eat all my lunch today, because I didn't have time.
Daddy: Uh-huh.
SK: It's true! I'm not even lying.
D: Oh, I believe you didn't eat all your lunch, don't worry about that.
SK: Ask Santa Claus if you think I'm lying.
D: Santa Claus?
SK: Yeah. Santa Claus actually can't see everyone all the time, but he's always watching.
D: OK.
SK: He has his house in... in... in the center of the world. But he has to move it around all the time. So he can watch everyone.
D: I guess that makes sense.
SK: And you know what? He used to live right here. In our house.
D: Really?
SK: Yeah.…
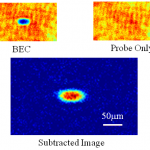
This is probably the last trip into the cold atom toolbox, unless I think of something else while I'm writing it. But don't make the mistake of assuming it's an afterthought-- far from it. In some ways, today's topic is the most important, because it covers the ways that we study the atoms once we have them trapped and cooled.
What do you mean? They're atoms, not Higgs bosons of something. You just... stick in a thermometer, or weigh them, or something... OK, actually, I have no idea. They're atoms, yes, but at ultra-low temperatures and in very small numbers. You can't bring them into…
Writing up the evaporative cooling post on cold atom techniques, I used the standard analogy that people in the field use for describing the process: cooling an atomic vapor to BEC is like the cooling of a cup of coffee, where the hottest component particles manage to escape the system of interest, and what's left behind is colder. The departing atoms or coffee molecules carry off more than the average energy per particle, leaving a lower average energy (and thus a lower temperature) for the remainder.
A question that sometimes comes up when I talk about this is how you can possibly use this…