Cold Atoms
I've been really busy with year-end wrap-up stuff, but have also posted a bunch of stuff at Forbes. which I've fallen down on my obligation to promote here... So, somewhat belatedly, here's a collection of physics-y stuff that I've written recently:
-- Using Atoms To Measure Tiny Forces: A post reporting on some very cool atom interferometry experiments, one working to measure the very tiny (but known to exist) force of gravity, the other searching for a possible "fifth force" sort of thing.
-- Making And Shaking New Materials With Ultracold Atoms: A post reporting on a couple more DAMOP…
I'm massively short on sleep today, and wasn't going to blog until I saw somebody on Facebook mention that June 5th 1995 is the date of record for the first Bose-Einstein condensate at JILA in Boulder. I couldn't let that pass, so I wrote it up for Forbes:
Twenty years ago, in the summer of 1995, I was a young grad student having just finished my second year at Maryland, and one morning I packed into the conference room at the National Institute of Standards and Technology (NIST) in Gaithersburg (where I worked in the group of Bill Phillips) with most of the rest of the Atomic Physics…
I'm rooting around in my bag for a pen, and pull out a laser pointer by mistake. Since I'd really prefer not to be grading, I flip it on and shine it on the floor next to the spot where Emmy is half-dozing. She immediately leaps up (she's pretty spry for a dog of 12...), and pounces on it. Or tries to, as I flick the spot across the room.
"Get the dot! Get the dot! Getthedotgetthedotgetthedot!" she mutters as I lead her on a lively chase around the room. After a few minutes, I click the laser off, and put it down. Emmy comes over, panting, and I scratch behind her ears.
"That was fun, eh,…
I wrote up another piece about football for the Conversation, this time drawing on material from Eureka, explaining how great football players are using scientific thinking:
Seattle Seahawks cornerback Richard Sherman gets called a lot of things. He calls himself the greatest cornerback in the NFL (and Seattle fans tend to agree). Sportswriters and some other players call him a loudmouth and a showboater. Fans of other teams call him a lot of things that shouldn’t see print (even on the internet). One thing you’re not likely to hear anyone on ESPN call Sherman, though, is “scientist.”
And…
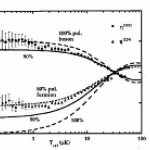
Topping the looooong list of things I would give a full ResearchBlogging write-up if I had time is this new paper on a ultra-cold atom realization of "Dirac Monopoles". This is really cool stuff, but there are a lot of intricacies that I don't fully understand, so writing it up isn't a simple matter.
The really short version, though, is that a team of AMO physicists have created particles that are analogous to magnetic monopoles-- that is, to a particle that was only a "north" or "south" pole of a magnet, not both together like a conventional bar magnet (leading to my favorite social-media…
Element: Ytterbium (Yb)
Atomic Number: 70
Mass: Seven "stable" isotopes, from 168 to 176 amu. Two of those are nominally radioactive, with half-lives vastly in excess of the age of the universe.
Laser cooling wavelength: 399 nm and 556 nm.
Doppler cooling limit: 690 μK in the UV and 4.4 μK in the green.
Chemical classification: A rare earth/ lanthanide, one of the hard to distinguish metals in the little island that floats off toward the bottom of the usual presentation of the periodic table, because it's too hard to wedge them in between barium and hafnium. Yet another greyish metal.
Other…
Element: Cesium (Cs)
Atomic Number: 55
Mass: One stable isotope, mass 133 amu.
Laser cooling wavelength: 854nm, but see below.
Doppler cooling limit: 125 μK.
Chemical classification: Yet another alkali metal, column I of the periodic table.
This one isn't greyish, though! It's kind of gold color. Still explodes violently in water, though.
Other properties of interest: The definition of the second in the SI system of units is in terms of the microwave transition between hyperfine ground states in Cs-- 9,192,631,770 oscillations to one second, to be precise. Has a really large scattering…
Element: Chromium (Cr)
Atomic Number: 24
Mass: Four "stable" isotopes between 50 and 54 amu. Chromium-50 is technically radioactive, with a half-life considerably longer than the age of the universe, so...
Laser cooling wavelength: 425nm, but see below.
Doppler cooling limit: 120 μK.
Chemical classification: Transition metal, smack in the middle of the periodic table. Shiny.
Other properties of interest: Has a fairly large magnetic moment in its ground state, 6 Bohr magnetons, which means it has strong magnetic interactions. For this reason, it's kind of an interesting system to study-- you…
Element: Lithium (Li)
Atomic Number: 3
Mass: Two stable isotopes, masses 6 and 7 amu
Laser cooling wavelength: 671 nm
Doppler cooling limit: 140 μK.
Chemical classification: Alkali metal, column I in the periodic table. Yet another greyish metal. We're almost done with alkalis, I promise. Less reactive than any of the others, so the explosions in water aren't very impressive.
Other properties of interest: Lithium-7 is a boson, but has a negative scattering length, meaning that BEC's of lithium-7 tend to implode unless you modify the collisional properties. Lithium-6 is a fermion, and much…
Element: Francium (Fr)
Atomic Number: 87
Mass: Numerous isotopes ranging in mass from 199 amu to 232 amu, none of them stable. The only ones laser cooled are the five between 208 amu and 212 amu, plus the one at 221 amu.
Laser cooling wavelength: 718 nm
Doppler cooling limit: 182 μK.
Chemical classification: Alkali metal, column I in the periodic table. The heaviest known alkali, it's presumably metallic in appearance, if you could ever get enough of it together to look at.
Other properties of interest: Francium has no stable isotopes, and the longest lifetime of any of its isotopes is around…
Element: Strontium (Sr)
Atomic Number: 38
Mass: Four stable isotopes, ranging from 84 to 88 amu
Laser cooling wavelength: Two different transitions are used in the laser cooling of strontium: a blue line at 461 nm that's an ordinary sort of transition, and an exceptionally narrow "intercombination" line at 689 nm.
Doppler cooling limit: 770 μK for the blue transition, below a microkelvin for the red. The Doppler limit for the red line turns out not to be all that relevant, as other factors significantly alter the cooling process.
Chemical classification: Alkaline earth, column II of the…
Element: Xenon (Xe)
Atomic Number: 54
Mass: nine "stable" isotopes, masses from 124 to 136 amu. Xenon-136 is technically radioactive, but with a half-life of a hundred billion billion years, so, you know, it's pretty much stable.
Laser cooling wavelength: 882 nm
Doppler cooling limit: 120 μK
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with anything, so poses much less danger to scientists than any of the alkalis, though it has, at times, been used as an anesthetic, and Will Happer's group at Princeton has a funny story about a student's…
Element: Helium (He)
Atomic Number: 2
Mass: two stable isotopes, 3 and 4 amu.
Laser cooling wavelength: 1083 nm
Doppler cooling limit: 38 μK
(It should be noted, though, that despite the low temperature, laser-cooled helium has a relatively high velocity-- that Doppler limit corresponds to an average velocity that's just about the same as for sodium at 240 μK. This is because temperature is a measure of kinetic energy, and helium is much, much lighter than any of the other laser-cooled elements.)
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with…
Element: Rubidium (Rb)
Atomic Number: 37
Mass: two "stable" isotopes, 85 and 87 amu (rubidium-87 is technically radioactive, but it's half-life is 48 billion years, so it might as well be stable for atomic physics purposes.
Laser cooling wavelength: 780 nm
Doppler cooling limit: 140 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it’s a greyish metal at room temperature. Like the other alkalis, it’s highly reactive, and bursts into flame on contact with water, even more so than sodium (in general, the alkalis get more violently reactive…
Element: Sodium (Na)
Atomic Number: 11
Mass: one stable isotope, 23 amu
Laser cooling wavelength: 589 nm
Doppler cooling limit: 240 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it's a greyish metal at room temperature. Like the other alkalis, it's highly reactive, and bursts into flame on contact with water. For this reason, all physicists working with sodium have True Lab Stories about accidentally blowing stuff up with it.
Other properties of interest: Scattering length of around 80 a0; Feshbach resonance at around 900 G.
History:…
At the tail end of the cold-atom toolbox series, I joked about doing a "trading card" version shortening the posts to a more web-friendly length. In idly thinking about this, though, it occurred to me that if one were going to have cold-atom trading cards, it might make more sense to have them for the atoms, rather than the techniques. And having just devoted many thousands of words to technique, I don't really feel like trying to cut those down more, but atoms...
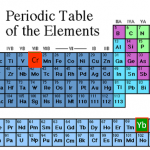
The "featured image" up top is a slide from my laser cooling lectures for our first-year seminar class. Elements outlined in red…
What's the application? Using lasers to reduce the speed of a sample of atoms, thereby reducing their temperature to a tiny fraction of a degree above absolute zero.
What problem(s) is it the solution to? 1) "How can I make this sample of atoms move slowly enough to measure their properties very accurately?" 2) "How can I make this sample of atoms move slowly enough for their quantum wave-like character to become apparent?"
How does it work? I've written about laser cooling before, but the nickel version of the explanation is this: You can think of a beam of light as being made up of photons…