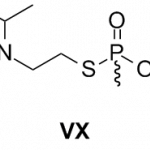poisons
A US Army base in Utah was locked down for some time on Wednesday, because they discovered that they (transiently) misplaced a container of VX - a potent chemical weapon. Like they used in The Rock!
A neurotransmitter called acetylcholine (or ACh) helps your muscles contract, as well as regulating functions as diverse as sweating, heart function, and pupil dilation. When everything is working correctly, ACh is a transient thing. It sends a message - for example, contract this muscle - and it is then broken down by an enzyme. It is important that, once it's done its job, ACh get broken down…
The latest news from the Gulf of Mexico offers both relief (the "top kill" approach to ending the oil spill may be working) and dismay (the amount of oil pouring into the water is now thought to be closer to 20,000 barrels a day rather than the 5,000 barrels that BP has insisted on for weeks.)
In other words - at worst case - the U.S. Geological Survey estimates that the spill amount may be closer to 39 million gallons of oil so far, rather than the 11 million previously suspected. Now, I've spent the last week or so focusing on the chemical dispersants used to break down the oil,…
Atropine can:
Dilate pupils
Speed the heart
Inhibit sweat and salivation
Serve as an "antidote" to "nerve gas"
Sounds like powerful medicine, and it is, indeed. On the other hand, it is named for Atropos, the Greek goddess responsible for deciding how people die, for a reason. This is a molecule best used by doctors, and only then with a surfeit of caution. Unfortunately, it seems to pop up everywhere!
The somewhat disparate effects of the compound come from its ability to block a particular neurotransmitter receptor. It has a lot of medical uses, due to the above effects.
However, as…
Last year, we were fretting about melamine contamination in foods from China. Again, this week, it's happening - melamine was put into milk by some unscrupulous vendors. The idea here is that melamine is high in nitrogen and cheap. An easy way to get an idea of how much protein is in something is to assay for nitrogen - by cutting milk with water and adding melamine, the unscrupulous can pass off watered down milk as full-strength.
It's become enough of a concern that it's hit US shores again and WHO is working on a test kit.
There's a very good chance it will involve cyanuric acid, which…
Sarin is an organophosphate that irreversibly inhibits cholinesterase. it's a neurotoxin, and a potent one. It'd be absolutely terrifying as a weapon, if it weren't so unstable. Even if a rogue state had gobs of Sarin last year, it's all pretty much a dud by now. The instability of acid halides (carbonyls there, but all of them in general) pretty much ensures this. So the civilized world has that going for it, which is nice.
How do you test for sarin leaks? With a rabbit.
Trying to think of a molecule tonight, my friend suggested "pick an ugly one no one wants anymore...a clearance rack molecule." I immediately went to chlorinated solvents. They're in the backwater now, right? Carbon tetrachloride sure has a bad rep. I figured most of the organochlorides, except for the ubiquitous lab solvents, would, too. I was wrong.
Perchloroethylene, or "perc," is a dry cleaning solvent. A few years back, I was surprised to learn that the non-chemistry nerds in my family knew of it, and I came to learn that grandpa owned a dry-cleaning shop back in the day, where he, in…
Acetaldehyde is an intermediate metabolite of alcohol:
It's the first stop for ethanol on the way to benign acetate. Aldehydes tend to be short-lived and toxic species because of their reactivity, and acetaldehyde is no different. On the way to your hangover, though, alcohol goes from ethanol to acetaldehyde (via alcohol dehydrogenase) to acetate (via aldehyde dehydrogenase). Many east Asians, interestingly, lack an adequate supply of aldehyde dehydrogenase, and they experience a sort of accelerated hangover on consumption of even moderate amounts of alcohol.
Gasoline and diesel engines operate using very different philosophies. In a gas engine, a spark ignites a compressed fuel-air mixture; in a diesel, air is compressed and gets very hot, and fuel is injected, resulting in ignition.
In the case of gasoline, the activation energy to start the fire is supplied by a high-energy spark. With diesel, the heat of compression must be sufficient to start the fire. For this reason, gas has an "octane number" (a measure of how difficult it is to ignite) and diesel has a "cetane number" (a measure of how easy it is to ignite). Low-octane gas can burn…
In the news the last couple days: chloropicrin:
Chloropicrin is a pesticide. It's one of the older types that's just generally really, really toxic. It will easily hydrolyze to phosgene and nitrosyl chloride, one of the nasties aqua regia generates.
Just recently, a Japanese farmer swallowed some in a suicide attempt, which resulted in toxic, phosgene and nitrosyl chloride-containing vomit, poisoning those around him.
This brought to mind a story which still gets to me, that of Jason Altom, Elias Corey's student who commited suicide nearly ten years ago with cyanide:
Having accounted for the…
The series of Breaking Bad chemicals continues (spoilers inside). Previously: phosphine, mercury fulminate. Today: hydrogen fluoride.
HF is just that, H-F, or a hydrogen bound to a fluorine. All the other acids in this group - HCl, HBr, HI - are gases. What we call "hydrochloric acid" is HCl in water, "hydrofluoric acid" is HF in water, etc. What's interesting, though, is that HCl, HBr, and HI are all strong acids - if you dissolve some in water, it will completely dissociate into H+ and Cl- (for example). HF doesn't do that, and that makes it unique.
While hydrochloric acid is strong stuff,…
Lots of chatter in the news today about finding ricin in a Vegas hotel room.
Image of PDB ID 2AAI from Wikipedia
Ricin works by inhibiting protein synthesis at the ribosomal level. This is a well-known method of killing something, and one on which many antibiotics work.
You'll note this looks different than most of my structures, and that's because ricin is a giant molecule in comparison - about 100 times larger than most of what you see here. It's a protein.
Proteins tend to be fragile things. Small molecules are a lot like jellybeans to proteins' gingerbread houses. Knock a jellybean off…
Aflatoxin is a toxin produced by certain funguses that acts on the liver:
The asperilligus strains that express aflatoxins (this is a broad class of molecules; the one above is aflatoxin B1) grow on lots of different crops. Your peanut butter certainly contains some. Fortunately, people are one of the hardiest species as far as aflatoxin exposure, and that PB&J is more a concern for someone with impaired liver function.
Ammonia, or NH3, is a mildly toxic gas (but not that bad - you use it in metabolism). Go down one, though, and you get more lavishly toxic.
Phosphine, or PH3, is much more toxic (go down another, to arsine, and you've got another toxic beastie). Phosphine is used in fumigation for this very reason.
Going down a group will reliably get you the bizzaro version of most chemicals. Methane, CH4, is an inert, flammable gas - silane, SiH4, isn't just flammable. It'll burn without any ignition source, going up in a puff of sand - thats right, not a whoosh of CO2 - SiO2 is the solid stuff sand and…
The previous entry on raspberry ketones got me thinking about supplements in general. For the most part they don't cause people problems - which is pretty remarkable, considering a lot of them do have real druglike molecules in them. Most people are taking these completely unsupervised, with only a label to guide them.
There's lots of toxic stuff in plants, too, though. One such compound is oxalic acid, which plants tolerate fine, mammals less so.
The big problem with oxalate is that it does a great job coordinating divalent metal cations, making insoluble complexes. Calcium oxalate can…
In many parts of the world, what Westerners would call "meat alternatives" are the main source of protein. One such product is tempeh, made from soy. A particular variety, when contaminated with a particular bacterium, can become contaminated with a potent toxin: bongkrek acid.
Bongkrek acid, like dinitrophenol and cyanide, is a mitochondrial poison.
Lewisite is nasty stuff - it's a compound of arsenic with two labile chlorines. As I mentioned yesterday, BAL is its antidote.
Lewisite is a chemical weapon from a particularly brutal era in this regard, and you hear about it a lot less these days. However, there's still plenty floating around...
Have a good weekend.
Strychnine is a well-known poison and detective novel trope with a moderately low LD50 (ca 10mg). You find it more often in NMRs these days.
NMR jocks love strychnine for some reason. It is a pretty good example of a molecule with a hard-to-solve structure that NMR quickly dispatches - see this PDF for some background. I don't get why it always seems to be around in NMR rooms, though - there's the rack of about 40 standards and forgotten samples (who is leaving all these tubes behind?), one of which contains enough strychnine for a decade of Agatha Christie novels - these are those…
Nitrobenzene is a simple enough molecule. It smells just like benzaldehyde - it's really strikingly similar if you've smelled both. If you haven't, it's the artificial almond-cherry flavoring smell.
Nitrobenzene, like many nitroaromatics, is a bit toxic (although not as bad as plain benzene, purportedly!) Its nice (sort of) smell has resulted in its use as a solvent. It used to be ubiquitous in shoe polish.
It also has some mild oxidizing character to it - I once did a reaction that involved dissolving your reagent in straight nitrobenzene and heating - it was both oxidizer and solvent (…
Cinnabar is an ore containing HgS, or mercury (II) sulfide:
It's a nice red color and pretty easy to get mercury out of - you just cook it, liberating the volatile sulfur and leaving behind mercury metal (volatile too; don't try this at home). It's funny that such a unique metal was isolated so easily.
Back with a real MoTD tomorrow!
Yesterday's entry on diethylene glycol reminded me of another public safety incident that occured pre-FDA: Jake leg.
Jamaican ginger extract, or "jake," was just like the extracts you buy at the grocery store today - full of alcohol. During prohibition, a lot of people realized it made a decent substitute for real booze. As far as I know, extracts can't use denatured alcohol - they're intended for consumption.
Soon, the treasury department wised up to this and declared that ginger extract had to have a certain amount of dissolved solids. Solomonic - if you wanted ginger extract, you were…