Last week, I wrote about a man named Jim Gass, a former chief legal counsel for Sylvania, who had suffered a debilitating stroke in 2009 that left him without the use of his left arm, and weak left leg. He could still walk with a cane, but was understandably desperate to try anything to be able to walk unaided and function more normally in life. Unfortunately (at least given what ultimately happened), Mr. Gass was both driven enough, credulous enough, and wealthy enough to spend $300,000 pursuing stem cell tourism in China, Mexico, and Argentina over the course of four years. The result is that he now has a tumor growing in his spinal column, as reported in The New England Journal of Medicine (NEJM) and The New York Times (NYT). Genetic analysis has demonstrated that the cells in this tumor mass did not come from Jim Gass, and the mass has left him paralyzed from the neck down, except for his right arm, incontinent, and with severe chronic back pain. Worse, although radiation temporarily stopped the tumor from growing, apparently it's growing again, and no one seems to know how to stop it. Given that the traits that make stem cells so desirable as a regenerative treatment, their plasticity and immortality (ability to divide indefinitely), are shared with cancer, scientists doing legitimate stem cell research have always feared such a complication and have therefore tried to take precautions to stop just this sort of thing from happening in clinical trials. Clearly, "stem cell tourist" clinics, which intentionally operate in countries where the regulatory environment is—shall we say?—less than rigorous are nowhere near as cautious.
At the time I wrote that article, I emphasized primarily clinics outside of the US, where shady operators locate in order to be able to operate largely unhindered by local governments. You'd think that such a thing couldn't possibly be going on in the US. You'd be wrong. About a week and a half ago, Paul Knoepfler, a stem cell scientist who maintains a blog about stem cells, teamed up with Leigh Turner to publish a paper in Cell Stem Cell estimating the number of stem cell clinics in the US. The number they came up with astonished me.
Stem cell clinics: A large and growing industry
In their article, "Selling Stem Cells in the USA: Assessing the Direct-to-Consumer Industry," Turner and Knoepfler explain:
Businesses marketing putative stem cell interventions have proliferated across the U.S. This commercial activity generates a host of serious ethical, scientific, legal, regulatory, and policy concerns. Perhaps the most obvious regulatory question is whether businesses advertising nonhomologous autologous, allogeneic, "induced pluripotent," or xenogeneic "stem cell therapies" are exposing their clients to noncompliant cell-based interventions. Such practices also prompt ethical concerns about the safety and efficacy of marketed interventions, accuracy in advertising, the quality of informed consent, and the exposure of vulnerable individuals to unjustifiable risks.
Prior analyses of companies engaged in direct-to-consumer marketing of stem cell interventions have not explicitly focused on attempting to comprehensively locate and examine U.S. businesses (Lau et al., 2008, Ogbogu et al., 2013, Regenberg et al., 2009), although recent scholarship has identified some U.S. businesses engaged in such activity (Connolly et al., 2014). While such companies have attracted some scrutiny from researchers and journalists, these businesses have not yet been examined in a comprehensive manner (Perrone, 2015, Turner, 2015a). This gap in scholarship has contributed to misunderstandings that need to be corrected.
For example, health researchers, policy-makers, patient advocacy groups, and reporters often use the phrase "stem cell tourism" when addressing the subject of unapproved cell-based interventions and even in 2016 assume that U.S. citizens must travel to such destinations as China, India, Mexico, and the Caribbean if they wish to access businesses promoting stem cell procedures for a wide range of clinical indications. While travel from the U.S. to international "stem cell clinics" continues, the rhetoric of "stem cell tourism" often fails to acknowledge the hundreds of U.S. businesses engaged in direct-to-consumer advertising of stem cell interventions.
Of course, I did exactly this in my previous post, not really acknowledging this industry in the US. True, I did mention the San Diego-based company Stemedica, but I mentioned that company mainly because its business model appears to involve doing actual FDA-monitored clinical trials of its stem cell products in the US but referring any patients contacting the company who are ineligible for its US clinical trials to one of its foreign partners, particularly its affiliate right across border in Tijuana, Novastem. It was, for example, Novastem through which Gordie Howe was treated for his stroke a year and a half ago. Patients referred to Stemedica's partners, of course, pay full price for the stem cell injections, usually around $30,000 a pop.
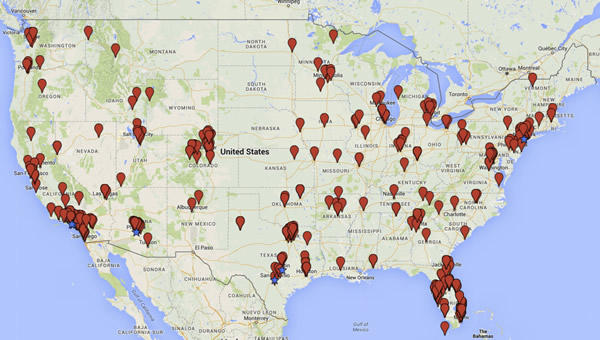
But what about US-based businesses? Turner and Knoepfler used key words and phrases such as "stem cell treatment" and "stem cell therapy" to preliminarily identify putative stem cell businesses and then evaluated the text on the websites of these businesses to refine their analysis. As a result, they identified 351 businesses offering stem cell therapies at 570 clinics, which they listed on this map:
They also helpfully include a link to an Excel spreadsheet listing all 570 sites, noting:
Many stem cell companies employ multiple physicians and advertise interventions available at numerous clinics. Although such businesses are widely distributed all over the county, we found that clinics tend to cluster in particular states. For example, we found 113 clinics in California, 104 in Florida, 71 in Texas, 37 in Colorado, 36 in Arizona, and 21 in New York. "Hotspot" cities including Beverly Hills (18), New York (14), San Antonio (13), Los Angeles (12), Austin (11), Scottsdale (11), and Phoenix (10) are designated with stars on the map. Some metropolitan areas, including Southern California around Los Angeles and San Diego, the South Florida region surrounding Miami, the greater Denver area, and the Dallas-Fort Worth metro region, have a relatively high number of clinics even if not all such facilities are technically in one city (Figure S1). While our analyses here do not explain why these businesses cluster in particular areas, we plan to investigate this question further. Possible factors include a relationship between number of clinics and population density, regional variations in use of "alternative" medical interventions, aging population demographics, and regulatory orientation of state medical boards and consumer protection agencies.
I'm sure population density has something to do with so many clinics in California, although one would expect more in New York and Texas if it were just population density. I also suspect that the prevalence and popularity of alternative medicine practitioners has something to do with it, since, oddly enough, I frequently see ads for stem cell clinics and articles praising stem cell therapies on websites oriented towards alternative medicine. Given how often stem cells are advertised as "anti-aging" treatments (something mentioned by Turner and Knoepfler) and the popularity of plastic surgery in California, it wouldn't surprise me if there is a correlation there as well. These are definitely things that I hope Turner and Knoepfler will look at in future investigations.
So what are these clinics selling stem cells to treat? What are the claims they make? Unfortunately, the claims of US clinics are not much, if at all, different from the claims made by many stem cell tourist clinics in other countries. Claims are made that specific diseases can be treated that are just specific enough to attract customers but vague enough not to promise too much.
Claims, claims, claims, and more claims
It has to be noted that there is not just one kind of stem cell. As I described last time, they range from embryonic stem cells that require human embryos to isolate, to adult stem cells, to cells induced to be stem cells by the introduction of genes responsible for maintaining the "stem cell" state. As I'll discuss later, this matters when it comes to asking just what the heck the FDA is or isn't doing about this proliferation of stem cell businesses.
Turner and Knoepfler note that most of the businesses that they identified market autologous stem cell-based interventions; i.e., stem cells isolated from the patient and then reinfused. Most isolated these stem cells (or claimed to isolate them, given that it's not always clear how such clinics verify that what they have isolated are indeed autologous stem cells) from adipose tissue (fat) or from the bone marrow. Be that as it may, 61% of the clinics examined market autologous adipose-derived stem cell-based interventions; 48% what they describe as autologous stem cells obtained from bone marrow; and 4% stem cells reportedly isolated from peripheral blood. Not surprisingly, lots of clinics offer stem cells isolated from more than one source. Some offer "mixed" stem cells from both bone marrow and adipose tissue as "combination stem cell therapy."
About one in five clinics advertised allogeneic stem cell treatments; i.e., stem cells from another person or source. The usual sources of these "stem cells" are advertised as amniotic material/fluid (17%), placenta (3.4%), and umbilical cords (0.6%). It's noted in the report the precise source for these products was not clear in all cases, in particular for amniotic stem cells. Indeed, one wonders (at least I do) what the source of amniotic fluid is from which these clinics claim to isolate stem cells. Do they have a deal with a local obstetrical clinic or hospital to provide amniotic fluid or membranes? Do they buy placentas and amniotic membranes from a hospital? Where do these clinics get the raw material (i.e., the human tissue and fluids) to generate these stem cells from? Inquiring minds want to know!
Turner and Knoepfler also noted one business that offers what it claims to be induced pluripotent stem cells. (Remember, these are cells genetically manipulated to revert to being stem cells.) I went back to the spreadsheet and found which company offered this, Regenerative Medical Group. RMG claims to provide "induced pluri-potent stem cells from your own cells via an affiliated laboratory," but what I found more interesting were the diseases and conditions it claims to treat with stem cells. Not surprisingly, as was the case for most of the clinics listed, many of the indications were orthopedic, to regenerate cartilage and repair injury. However, RMG also claims to be able to treat kidney diseases, macular degeneration, Parkinson's disease, and, yes, autism. Under a tagline of An autism therapy that WORKS, there's even a video on the website that makes claims that can only be described as grandiose and not supported by science featuring Bryn J. Henderson, DO, JD, FACPE, CIME, the executive director of RMG:
In the video, Dr. Henderson claims that RMG has helped "dozens" of children with autism using stem cells. He claims that the stem cells circulate through the body, cross the blood-brain barrier and "make new cells" that change the course and prognosis of the patient with autism. He even claims that most of the time, the change is major. How does he know? He brags about the thank you cards he's gotten from parents. I mean, seriously. This is utterly pathetic. Even antivaccine quacks like Mark and David Geier or Andrew Wakefield can do better at providing "evidence". Note that that is not a compliment, given how poor their attempts at studies invariably are. Dr. Henderson, however, presents no science, no clinical trials, no preclinical trials, no nothing other than testimonials, although he does use a lot of science-y-sounding terms. Hell, I've seen homeopaths who provide more evidence and a more convincing presentation. At least they will cite actual patients rather than thank you notes from patients' families. (Oh, and Dr. Henderson, don't bother taking your video down; I've downloaded it.)
RMG's fact sheet on autism is no better. Citing no evidence, not even case reports, the sheet claims that stem cell infusions for autism can improve:
- Social interaction
- Sensory problems
- Seizures
- Mental health issues
- Mental impairment
- Digestive issues
- Sleep disturbances
- Aggression
What evidence is presented? Again, none. This might as well be a chelation therapy clinic. The treatment, however, takes three days, and the patient doesn't have to come back to an RMG clinic at all, although, the fact sheet hastens to add, they can undergo repeat treatments if necessary. In other words, stem cells appear to have been added to the armamentarium of "autism biomed" quackery.
I could go on, but to me stem cells for autism is so obviously dubious at best and bogus at worst that, given my interest in vaccines and the antivaccine movement's mistaken belief that vaccines cause autism, I hope you'll forgive me if I zeroed right in on autism. Indeed, nine of the clinics listed in the spreadsheet claim to be able to use stem cells of one kind or another to treat autism.
But, wait, there's more. In addition to RMG:
Another business markets access to what it describes as "embryonic stem cell" interventions. In addition, we identified two clinics that marketed "bovine amniotic cells," a xenogeneic product, for use in humans. Approximately 3% of businesses marketed stem cell interventions without mentioning a particular type of stem cells.
Perusing the list of clinics, I found it hard not to come to the conclusion that there isn't a single disease or condition that someone, somewhere, isn't claiming can be helped with stem cells of one kind or another. Diabetes, heart disease, degenerative diseases, Parkinson's disease, Alzheimer's disease, spinal cord injuries, stroke, aging, and even cancer show up on the list of conditions that these 570 clinics claim to be able to treat with stem cells, as Turner and Knoepfler note:
U.S. businesses promoting stem cell interventions claim to treat a wide range of diseases and injuries, as well as advertising stem cells for cosmetic applications, "anti-aging," and other purposes (Figure 2B). Some clinics occupy relatively specialized marketplace niches. For example, many cosmetic surgery clinics advertise such procedures as "stem cell facelifts" and "stem cell breast augmentation" as well as sexual enhancement procedures. Orthopedic and sports medicine clinics often promote stem cell interventions for joints and soft tissue injuries. Other clinics take a much broader approach and list stem cell interventions for 30 or more diseases and injuries. Such businesses commonly market treatments for neurological disorders and other degenerative conditions, spinal cord injuries, immunological conditions, cardiac diseases, pulmonary disorders, ophthalmological diseases and injuries, and urological diseases as well as cosmetic indications. Many of these marketing claims raise significant ethical issues given the lack of peer-reviewed evidence that advertised stem cell interventions are safe and efficacious for the treatment of particular diseases. Such promotional claims also generate regulatory concerns due to apparent noncompliance with federal regulations.
Unfortunately, these US businesses are less unlike the stem cell tourist clinics that I've written about before than I would like.
Where's the FDA?
I thought about perusing the list of clinics in more detail and picking out the most egregious examples other than RMG, but that can wait for a potential future post. (It is, after all, a holiday weekend, and we'll be having visitors.) So instead I'll move on to conclude with the question that many of you are probably wondering after seeing an example such as treating autism with stem cells: What the heck is the FDA doing?
This isn't as simple as it sounds. For one thing, as noted in Turner and Knoepfler's supplemental methods section:
However, it should be noted that according to 21 CFR 1271.3 (d) (4), minimally manipulated bone marrow for homologous use does not require pre-marketing approval by the FDA. 21 CFR 1271.15 (b) states that facilities removing cells or tissues from an individual and implanting those cells or tissues in the same individual during the same surgical procedure likewise do not require premarketing approval. In addition, federal regulations contain detailed criteria specifying when autologous or allogeneic cells can be used without first obtaining FDA premarketing approval. These criteria are identified in 21 CFR 1271.10. We mention these important sections of 21 CFR 1271 for a reason. Our goal was to identify businesses that engage in direct-to-consumer marketing of stem cell interventions and fit within our inclusion criteria. Judgments about regulatory compliance or noncompliance had no bearing on whether specific businesses were included in our database. Federal regulations governing marketing, manufacture, administration, and registration of cell-based interventions are complex, products are classified into different risk- based regulatory tiers, and we in no way wish to claim or imply that inclusion of particular businesses in Supplemental Table 1 means that they are noncompliant with federal regulations. Such determinations, as well as other assessments of regulatory compliance, must be made by legally authorized regulatory agencies after rigorous evaluation processes.
This is, of course, the reason why so many of these businesses offer—or claim to offer—bone marrow or adipose stem cells. If they don't manipulate the cells too much, they can skirt FDA regulations, although the FDA is moving to crack down on unproven stem cell treatments and have started to issue warning letters. It's a complex issue, but it's hard not to look at the number of clinics and the breadth of health claims documented by Turner and Knoepfler and not come to the conclusion that there is a serious problem here. It's also clear that big money and political interests are hindering the FDA. For example:
Some proponents of deregulation argue that current federal regulations governing the advertising, processing, and administration of autologous stem cells are too onerous and have resulted in few approved stem cell therapies reaching the American marketplace (Chirba and Garfield, 2011, McAllister et al., 2012). The REGROW Act is an example of the current push from some political quarters and even from some individual stem cell researchers for lowering safety and efficacy standards for adult stem cell-based interventions. However, we found that hundreds of U.S. businesses are already promoting stem cell interventions for an extraordinary range of clinical indications. Advocates of deregulation will perhaps be pleased by our findings that many putative stem cell interventions are currently available for sale in the U.S. In contrast, proponents of a marketplace in which cell-based therapies have traditionally been tested for safety and efficacy and subject to pre-marketing review by the FDA will likely be concerned by how many U.S. businesses are currently marketing stem cell interventions. We are particularly concerned that we found many advertising claims related to ALS, Alzheimer's disease, Parkinson's disease, and many other conditions for which there is no established scientific consensus that proven safe and efficacious stem cell treatments now exist.
The REGROW Act sounds a lot like the 21st Century Cures Act, ideologically-driven "solutions" that mistakenly argue that the way to let loose a torrent of cures for every disease imaginable is to "unleash" the power of the market through deregulation. In the case of the 21st Century Cures Act, its proponents propose to give the NIH a bit more money in return for weakening the FDA. It's basically a solution to a nonexistent problem. The REGROW Act is cut from the same cloth, as it would allow provisional approval of stem cell therapies without phase III trials and establishing a conditional approval paradigm. Together with "right to try" laws, the REGROW Act and the 21st Century Cures Act are of a piece with a libertarian, free market-driven agenda to hamper government regulatory agencies. Fortunately, the the REGROW Act v.2.0 appears to be going nowhere fast. Meanwhile these stem cell clinics are scrambling to deny that they are doing anything unethical, illegal, or dangerous.
Perusing some of the websites, I couldn't help but notice how dubious stem cell therapies seem to have found a comfortable home in alternative medicine clinics. Perhaps the most blatant example I found was the Purety Family Medical Clinic, which advertises itself as "holistic medicine specialists for women, men, and pediatrics as well as prolotherapy, IV, ozone, chelation, HRT and FMT." Right alongside stem cell injections for "badly injured or degenerated tissue," Purety also offers chelation therapy for "heavy metal detoxification," high dose vitamin C drips, ozone therapy for cancer, naturopathy, fecal transplants for a variety of illnesses, and, yes, homeopathy, The One Quackery to Rule Them All.
Unfortunately, given how potentially promising stem cell therapies are, right now they are tainted by association with quackery like that described above. Basically, stem cells are being sold as being every bit as magical as alternative medicine like homeopathy. However, as PZ Myers points out:
Stem cells are not magic. They are plastic cells that are pluripotent — they can differentiate into a variety of different tissues. But they need instructions and signals in order to develop in a constructive way, and the hard part is reconstructing environmental cues to shape their actions. They're like Lego building blocks — you can build model spaceships or submarines or houses with them, and they have a lot of creative potential, but it's not enough to just throw the Lego blocks into a bag and shake them really hard.
That's what these stem cell clinics are doing, injecting stem cells and hoping they do their thing without knowing how the body induces them to do their thing, all while charging patients large sums of money for the privilege of being in what is in essence a poorly designed, poorly regulated clinical trial.
I don't know about you, but if I were a legitimate advocate of stem cell therapies, I'd be very disturbed at how easily stem cell therapies are currently "integrated" with pure quackery like chelation therapy and homeopathy. Being so easily associated with clinics like Purety is not a good way to make stem cell treatments respectable, but it is a good way to make a lot of money if you aren't that concerned with medical evidence or ethics—at least until the next Jim Gass hits the news.

Bad enough for these people to prey upon desperate adults; it's even worse that they're pushing an unproven, potentially dangerous therapy on children.
Turner and Knoepfler will need to update their map to show an additional stem cell clinic in Ohio:
http://buckeyephysicalmedicine.com/
These folks appear to treat virtually any kind of musculoskeletal pain with stem cell therapy, though it is odd that I can't find the names of any of their "medical professional" staff on the website. However, this operation appears to be part of a chiropractic practice featuring someone known as "Dr. Buzz" (they share addresses).
Not sure how and why chiros are permitted to employ such treatment, this is certainly not the only such operation out there.
These factors go a long way to explaining why there are so many clinics in Beverly Hills alone--half again as many as in the much larger neighboring city of Los Angeles. The availability of money certainly helps.
But other locations are definitely headscratchers. For instance, why is there such a cluster along Colorado's Front Range? You also see some clinics in out-of-the-way places like the Iron Range region of Minnesota or a truck stop town along I-80 in northern Nevada. Certainly there are people in Colorado who have money (the clinics in the mountain resort towns cater to that crowd), but the other two are just out there.
From the map it looks like there aren't any in my home state of New Hampshire. I'd have to drive to Boston, Providence, Burlington, or New Haven. But I'm not complaining about that.
Although there seem to be markers on my city on the map, I could not find any locations in my city or state on the spreadsheet--thank goodness! I did notice a glut of them in my former states-- woo paradises. All are in higher income locations.
And be sure to let Harriet Hall know there is one in Puyallup!
And that right away puts it in the "no way, no how" bucket for me as a treatment for autism. Or just about anything else. I mean, that's basically saying "this could do huge, massive, horrendous, awful things to do" so now he's got the burden of convincing me that it won't. But like all these stem cell therapy promotional materials, it doesn't even try. It just assures me that somehow it will only have positive effects.
Make new cells? So? I mean, even the most basic layperson should be wondering "so, what makes it be the *right* cells, doing the *right* thing?" I don't need liver cells in my brain, or brain cells in my earlobe. Or worse. I mean seriously, their promotional materials sound terrifying. If it does what he claims, then it should be incredibly dangerous.
And, of course, it is. Damn. This stuff's seriously scary.
As I sometimes say in other contexts, the Law of Unintended Consequences is strictly enforced. As the case of Mr. Gass shows, that's also the case here.
I've got an update for the spreadsheet at well.
It has a very similar name to column 148 (Manhattan Integrative Medicine Medical Group), going by the name of Manhattan Integrative Medicine. It's run by a guy named Dr. David Borenstein who's been interviewed on Faux News about his stem cell treatments for COPD, heart disease and a host of other stuff.
I stumbled across his website doing research for an assignment in my Pathophysiology class. I almost emailed Orac to ask him if he'd ever heard of this sort of thing in the US.
I'm very sorry to find out just how much of it there is :'(
Quack therapies on children are doubly disturbing due to a child's inability to consent for treatment. That a physician is "treating" autism by reinjecting cells taken from a child and modified in unspecified/untested/unproven ways is incredibly disturbing. I'd like to think there's at least a few people at the FDA who are as incredulous at this and also somehow have the power and ability to take action to stop this.
Stem cells “make new cells” that change the course of autism if the patients religiously.use an IonCleanse foot bath, which detoxes the nasty new cells that plagued Mr. Gass, and sends positive ionic pulses that guide the new cells across the blood-brain barrier in just the right places! [Clinical trial forthcoming.]
I missing several critical pieces of cartilage and would love to have those pieces regenerated from autologous stem cells -- a relatively simple structure and application -- but I'm concerned about the long term effect of turning relatively uncontrolled cells loose in my body. I'm watching the legitimate trials, but I may opt for titanium anyway.
If anything, I'd say this is worse than chelation therapy - at least chelation therapy is based on a proposed mechanism (e.g., that autism is a form of mercury poisoning.) If you were feeling really generous you might even call it a somewhat plausible mechanism, if you kinda squint and look at it out of the corner of your eye in a dim light. But as far as I can tell these stem cell "clinics" don't even bother trying to come up with an explanation for how stem cells are supposed to treat autism. It's not as if autism is caused by a lack of brain cells, which is the only possible reason I can think of to try and treat it with stem cells. In fact, IIRC, one of the major differences between autistic and neurotypical brains is that autistics have more neural connections - it's the "pruning" function that's faulty.
Incidentally, if you should happen to need blogging material in the near future this article was in my university newsfeed this morning. The original study is here; it's a case-control study that found that getting all three doses of the HPV vaccine decreases the risk of having an abnormal Pap smear, especially "high grade" abnormalities. It's not what you'd call "slam dunk" evidence but the article does a pretty good job of putting the results in context.
@sadmar #9:
No, no, no! Only the HeavenSent Deep InfraRed Sauna can detox all the cells correctly. What are you trying to do, kill children?
At least if you survive the administration of the chelator, you don't have out-of-control cells growing wherever they happen to have been injected.
@ Eric Lund:
I notice clusters of clinics near LA, SF and NYC which certainly may be due to the money coagulated there.
I'm less sure about Florida though- clinics ring the coast - places rich and not-so-rich.
Stella B @10: That's what I kept trying to explain to my MIL - they say that the stem cells will grow you new cartilage, but it could just as well grow fat or bone, and growing bone inside your knee would be much, much worse than how it is now.
I'm glad she changed her mind about that.
@Denice: Florida has something of a reputation as an alt-med hotbed (at least one person profiled more than once in the years I have been reading this blog has his home base there, and IINM some other alt-med gurus have homes in the state). Florida also has lots of retirees who may be easy marks for anti-aging pitches, and many of those retirees have disposable assets. The clinics are on the coasts and along the I-4 corridor because those are the parts of the state where most of the people live. I'll grant that Key West is an odd location, though.
Ft. Collins is home to a state university with a big emphasis on animal research, which I imagine includes stem cell research. There have been big biotech firms in the area, so I think our quacks borrow their vocabulary and blend in.
And then, there's the energy vortex in Boulder....
The G—le ads suggest that Dr. Kevin Darr of Covington, LA, may have been overlooked as well.
This is starting to remind me of the plot of a short film called "The Gate" that can be watched on YouTube (link below). People purchasing alleged 'miracle drugs' from virtually unregulated quacks without any thought for the consequences.
https://www.youtube.com/watch?v=yqyQmecxub4
From the article: "This might as well be a chelation therapy clinic"
Gotta blow the whistle on this one. Chelation is the only proven way, that I know of, of reversing symptoms of Lead and Mercury Poisoning. The chemistry is well understood for many compounds, and EDTA has FDA-approval for treating Lead Poisoning.
DMSA is effective for Mercury Chelation: http://www.ncbi.nlm.nih.gov/pubmed/9630737
This does not belong in the "woo" category.
Billy Rubin, while chelation is a genuine method for removing heavy metals from a body, the Chelation Clinics referred to here are the quack ones set up to extract money from parents of kids with autism under the disproven belief that heavy metals, specifically mercury, causes autism. Often diagnosed by other allied quack labs using techniques that will amplify the normal amounts of such chemicals present in the average human body, e.g. if you eat tuna at all you will have some mercury in your system as you likely will if you live downwind of a coal fired power station or your water source does, to make it look as if they have high levels when they don't.
Stem-cell-quackery death in Australia using fraudulent technology sourced from the US:
http://www.abc.net.au/news/2014-09-16/stem-cell-methods-doctor-ralph-br…
I also wonder.. even going by their own logic.. what is the benefit of just re-implanting stem cells that are already in your body!?
If they were meant to regenerate an specific tissue the local stem cell population normally has been movilized already