Atoms and Molecules
Two papers with a similar theme crossed my social media feeds in the last couple of days. You might think this is just a weird coincidence, but I'm choosing to take it as a sign to write about them for the blog.
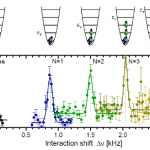
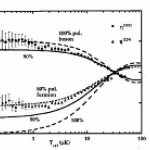
So, what are these papers, and what's the theme? One is the final publication of some results I saw at DAMOP and alluded to back in June, and the other is from this post by Doug Natelson. Both look at the transition from few-body to many-body physics.
And this is interesting, why? I mean, isn't it obvious that you just add some more bodies? OK, I guess that does need a little more…
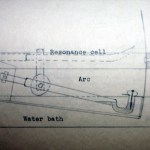
Having spent a bunch of time talking about heavy stuff in the science blogging community, let's unwind a bit and kick the week off with a look back at an old Master's thesis. This one is from 1932, and is almost certainly a draft copy, because it's extremely cheaply bound in cardboard with the title hand-lettered on the front. There are a few corrections in the text as well, which is very short-- just 11 pages, not counting the figures at the end.
Again, this is interesting as much for what it doesn't contain as what it does. The subject matter is pretty mundane by modern standards-- just…
As noted in a previous post on Monte Carlo simulation in 1960, we recently came into possession of a large box of old Master's theses. The bulk of these are from the 50's and 60's, but there are some going back much farther. As I pass these every day I'm in the office, I thought it might be amusing to take a look at these for the blog, now and again. I don't plan to do a detailed examination of the quality of the science (at least, not necessarily), but to use this to look at how things have changed over the decades.
The first of these, pictured above, is one of the oldest: Secondary Emission…
Through some kind of weird synchronicity, the title question came up twice yesterday, once in a comment to my TED@NYC talk post, and the second time on Twitter, in a conversation with a person whose account is protected, thus rendering it un-link-able. Trust me.
The question is one of those things that you don't necessarily think about right off-- of course an atom is a particle!-- but once it gets brought up, you realize it's a little subtle. Because, after all, while electrons and photons are fundamental particles, with no internal structure, atoms are made of smaller things. But somehow we…
The following is the approximate text of the talk I gave at TED@NYC last night. Approximate, because I'm somewhat prone to ad-libbing when speaking, and may have changed a few things here and there. I don't really know, because I'm scheduling this post on Tuesday morning, before the actual event, using the draft text I've been rehearsing with. But this will give you something to read while I drive back home from The City, and I can provide a more detailed recap later.
----
I’m going to tell you the most amazing thing I know, which is this: everything in the universe, from light, to electrons…
I'm putting together slides for a TED audition talk in a couple of weeks, about how the history of quantum mechanics is like a crossword puzzle. This involves talking about black-body radiation, which is the problem that kicked off QM-- to explain the spectrum of light emitted by hot objects, Max Planck had to resort to a mathematical trick: he assumed that the objects were composed of "oscillators" that emitted light in discrete amounts, with the energy of the emitted light proportional to the frequency of the light. This was a desperation move, and made him a little crazy:
Max Planck…
Element: Ytterbium (Yb)
Atomic Number: 70
Mass: Seven "stable" isotopes, from 168 to 176 amu. Two of those are nominally radioactive, with half-lives vastly in excess of the age of the universe.
Laser cooling wavelength: 399 nm and 556 nm.
Doppler cooling limit: 690 μK in the UV and 4.4 μK in the green.
Chemical classification: A rare earth/ lanthanide, one of the hard to distinguish metals in the little island that floats off toward the bottom of the usual presentation of the periodic table, because it's too hard to wedge them in between barium and hafnium. Yet another greyish metal.
Other…
Two chapters of the book-in-progress will be devoted to the development of the modern understanding of the atom. One of these is about the Bohr model, which turned 100 this year, but Bohr's model would not have been possible without an earlier experiment. The actual experiment was done by Ernest Marsden and Hans Geiger, but as is the way of such things, the historical credit mostly accrues to their boss and noted force of nature, Ernest Rutherford. This is the experiment that established the cartoon image of an atom as a solar system, which is utterly unworkable using classical physics, and…
Element: Cesium (Cs)
Atomic Number: 55
Mass: One stable isotope, mass 133 amu.
Laser cooling wavelength: 854nm, but see below.
Doppler cooling limit: 125 μK.
Chemical classification: Yet another alkali metal, column I of the periodic table.
This one isn't greyish, though! It's kind of gold color. Still explodes violently in water, though.
Other properties of interest: The definition of the second in the SI system of units is in terms of the microwave transition between hyperfine ground states in Cs-- 9,192,631,770 oscillations to one second, to be precise. Has a really large scattering…
Element: Chromium (Cr)
Atomic Number: 24
Mass: Four "stable" isotopes between 50 and 54 amu. Chromium-50 is technically radioactive, with a half-life considerably longer than the age of the universe, so...
Laser cooling wavelength: 425nm, but see below.
Doppler cooling limit: 120 μK.
Chemical classification: Transition metal, smack in the middle of the periodic table. Shiny.
Other properties of interest: Has a fairly large magnetic moment in its ground state, 6 Bohr magnetons, which means it has strong magnetic interactions. For this reason, it's kind of an interesting system to study-- you…
Element: Lithium (Li)
Atomic Number: 3
Mass: Two stable isotopes, masses 6 and 7 amu
Laser cooling wavelength: 671 nm
Doppler cooling limit: 140 μK.
Chemical classification: Alkali metal, column I in the periodic table. Yet another greyish metal. We're almost done with alkalis, I promise. Less reactive than any of the others, so the explosions in water aren't very impressive.
Other properties of interest: Lithium-7 is a boson, but has a negative scattering length, meaning that BEC's of lithium-7 tend to implode unless you modify the collisional properties. Lithium-6 is a fermion, and much…
Element: Francium (Fr)
Atomic Number: 87
Mass: Numerous isotopes ranging in mass from 199 amu to 232 amu, none of them stable. The only ones laser cooled are the five between 208 amu and 212 amu, plus the one at 221 amu.
Laser cooling wavelength: 718 nm
Doppler cooling limit: 182 μK.
Chemical classification: Alkali metal, column I in the periodic table. The heaviest known alkali, it's presumably metallic in appearance, if you could ever get enough of it together to look at.
Other properties of interest: Francium has no stable isotopes, and the longest lifetime of any of its isotopes is around…
Element: Strontium (Sr)
Atomic Number: 38
Mass: Four stable isotopes, ranging from 84 to 88 amu
Laser cooling wavelength: Two different transitions are used in the laser cooling of strontium: a blue line at 461 nm that's an ordinary sort of transition, and an exceptionally narrow "intercombination" line at 689 nm.
Doppler cooling limit: 770 μK for the blue transition, below a microkelvin for the red. The Doppler limit for the red line turns out not to be all that relevant, as other factors significantly alter the cooling process.
Chemical classification: Alkaline earth, column II of the…
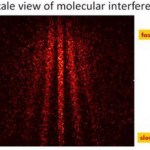
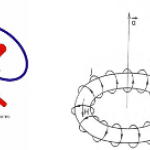
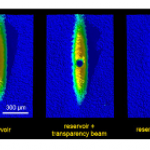
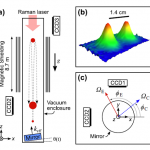
When I wrote up the giant interferometer experiment at Stanford, I noted that they've managed to create a situation where the wavefunction of the atoms passing through their interferometer contains two peaks separated by almost a centimeter and a half. This isn't two clouds of atoms each definitely in a particular position, mind, this is a wavefunction representing a bunch of atoms that are each partly in two places at the same time , separated by 1.4 centimeters.
I emailed Mark a link to the post, and in his reply he said that they've increased that to about 4cm (which is just a matter of…
Element: Xenon (Xe)
Atomic Number: 54
Mass: nine "stable" isotopes, masses from 124 to 136 amu. Xenon-136 is technically radioactive, but with a half-life of a hundred billion billion years, so, you know, it's pretty much stable.
Laser cooling wavelength: 882 nm
Doppler cooling limit: 120 μK
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with anything, so poses much less danger to scientists than any of the alkalis, though it has, at times, been used as an anesthetic, and Will Happer's group at Princeton has a funny story about a student's…
Element: Helium (He)
Atomic Number: 2
Mass: two stable isotopes, 3 and 4 amu.
Laser cooling wavelength: 1083 nm
Doppler cooling limit: 38 μK
(It should be noted, though, that despite the low temperature, laser-cooled helium has a relatively high velocity-- that Doppler limit corresponds to an average velocity that's just about the same as for sodium at 240 μK. This is because temperature is a measure of kinetic energy, and helium is much, much lighter than any of the other laser-cooled elements.)
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with…
A little over a year ago, I visited Mark Kasevich's labs at Stanford, and wrote up a paper proposing to use a 10-m atom interferometer to test general relativity. Now, that sounds crazy, but I saw the actual tower when I visited, so it wasn't complete nonsense. And this week, they have a new paper with experimental results, that's free to read via this Physics Focus article. Which might seem to make me blogging it redundant, but I think it's cool enough that I can't resist.
OK, dude, "Multiaxis Inertial Sensing with Long-Time Point Source Atom Interferometry" is not the sexiest title in the…
Element: Rubidium (Rb)
Atomic Number: 37
Mass: two "stable" isotopes, 85 and 87 amu (rubidium-87 is technically radioactive, but it's half-life is 48 billion years, so it might as well be stable for atomic physics purposes.
Laser cooling wavelength: 780 nm
Doppler cooling limit: 140 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it’s a greyish metal at room temperature. Like the other alkalis, it’s highly reactive, and bursts into flame on contact with water, even more so than sodium (in general, the alkalis get more violently reactive…
Element: Sodium (Na)
Atomic Number: 11
Mass: one stable isotope, 23 amu
Laser cooling wavelength: 589 nm
Doppler cooling limit: 240 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it's a greyish metal at room temperature. Like the other alkalis, it's highly reactive, and bursts into flame on contact with water. For this reason, all physicists working with sodium have True Lab Stories about accidentally blowing stuff up with it.
Other properties of interest: Scattering length of around 80 a0; Feshbach resonance at around 900 G.
History:…
At the tail end of the cold-atom toolbox series, I joked about doing a "trading card" version shortening the posts to a more web-friendly length. In idly thinking about this, though, it occurred to me that if one were going to have cold-atom trading cards, it might make more sense to have them for the atoms, rather than the techniques. And having just devoted many thousands of words to technique, I don't really feel like trying to cut those down more, but atoms...
The "featured image" up top is a slide from my laser cooling lectures for our first-year seminar class. Elements outlined in red…