Lasers
I'm rooting around in my bag for a pen, and pull out a laser pointer by mistake. Since I'd really prefer not to be grading, I flip it on and shine it on the floor next to the spot where Emmy is half-dozing. She immediately leaps up (she's pretty spry for a dog of 12...), and pounces on it. Or tries to, as I flick the spot across the room.
"Get the dot! Get the dot! Getthedotgetthedotgetthedot!" she mutters as I lead her on a lively chase around the room. After a few minutes, I click the laser off, and put it down. Emmy comes over, panting, and I scratch behind her ears.
"That was fun, eh,…
The 2014 Nobel Prize in Physics has been awarded to Isamu Akasaki, Hiroshi Amano and Shuji Nakamura for the development of blue LED's. As always, this is kind of fascinating to watch evolve in the social media sphere, because as a genuinely unexpected big science story, journalists don't have pre-written articles based on an early copy of a embargoed paper. Which means absolutely everybody starts out using almost the exact words of the official Nobel press release, because that fills space while they frantically research the subject. Later in the day, you'll get some different framing, once…
In a weird coincidence, shortly after I wrote a post about "quantum leap" as a metaphor, I was looking up some stuff about John Bell and ran into mentions of a paper he wrote called "Are There Quantum Jumps?" Bell is borrowing a title from Schrödinger, who wrote a pair of articles (really, one article in two pieces) for the British Journal for the Philosophy of Science in 1952 expressing his discontent with the entire idea of "quantum jumps" between states. Bell even opens his paper with a quote from Schrödinger: "If we have to go on with these damned quantum jumps, then I'm sorry that I ever…
On Twitter Sunday morning, the National Society of Black Physicsts account retweeted this:
Using Lasers to Lock Down #Exoplanet Hunting #Space
http://t.co/0TN4DDo7LF
— ✨The Solar System✨ (@The_SolarSystem) September 28, 2014
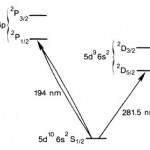
I recognized the title as a likely reference to the use of optical frequency combs as calibration sources for spectrometry, which is awesome stuff. Unfortunately, the story at that link is less awesome than awful. It goes on at some length about the astronomy, then dispenses with the physics in two short paragraphs of joking references to scare-quoted jargon from the…
As our planet makes more and more noise, we can't help but wonder why no one is paying attention. Are we alone in the universe? Or alone in our desire to discover new worlds? PZ Myers says "Spaceship building is never going to be a selectively advantageous feature — it’s only going to emerge as a spandrel, which might lead to a species that can occupy a novel niche." Humanity could tread that path, following our dreams to the stars. But even then, we might only find extraterrestrials in the form of well-adjusted slime blobs, content in their otherworldly ecosystems.
If there are other tech-…
I didn't plan to do a follow-up to yesterday's post about the optics of sending messages with lasers, but then I starting idly thinking about detection, prompted in part by a bunch of conversations with my summer students about single-photon detectors. which led to scribbling on the back of an envelope, which led to Googling, and suddenly, I have a follow-up post.
So: as we said yesterday, if you want to send messages over a distance of ten light years, a relatively efficient way to do this might be to send them via lasers. This results in the light being spread over a pretty big area, though…
In the comments to yesterday's grumpy post about the Fermi paradox, makeinu raises the idea that advanced aliens would be using more targeted communications than we do:
On the point about electromagnetic communications: even we are now using lasers to target communications with space, because it’s simply more efficient and reliable.
It’s also basically impossible to intercept, since you literally have to interrupt the beam to do so.
This is true, to a point, but when you're talking about interstellar distances, it's not quite true that you have to interrupt the beam to detect communications…
Perovskite solar cells can not only emit light, they can also emit up to 70% of absorbed sunlight as lasers.
Critical signaling molecules can be used to convert stem cells to neural progenitor cells, increasing the yield of healthy motor neurons and decreasing the time required to grow them.
Mexican blind cavefish are so close to their sighted kin that they are considered the same species, but they use pressure waves (from opening and closing their mouths) to navigate in the dark.
Electrostatic assembly allows luminescent elements (like Europium) to be embedded in nanodiamonds; these glowing…
Element: Ytterbium (Yb)
Atomic Number: 70
Mass: Seven "stable" isotopes, from 168 to 176 amu. Two of those are nominally radioactive, with half-lives vastly in excess of the age of the universe.
Laser cooling wavelength: 399 nm and 556 nm.
Doppler cooling limit: 690 μK in the UV and 4.4 μK in the green.
Chemical classification: A rare earth/ lanthanide, one of the hard to distinguish metals in the little island that floats off toward the bottom of the usual presentation of the periodic table, because it's too hard to wedge them in between barium and hafnium. Yet another greyish metal.
Other…
Element: Cesium (Cs)
Atomic Number: 55
Mass: One stable isotope, mass 133 amu.
Laser cooling wavelength: 854nm, but see below.
Doppler cooling limit: 125 μK.
Chemical classification: Yet another alkali metal, column I of the periodic table.
This one isn't greyish, though! It's kind of gold color. Still explodes violently in water, though.
Other properties of interest: The definition of the second in the SI system of units is in terms of the microwave transition between hyperfine ground states in Cs-- 9,192,631,770 oscillations to one second, to be precise. Has a really large scattering…
Element: Chromium (Cr)
Atomic Number: 24
Mass: Four "stable" isotopes between 50 and 54 amu. Chromium-50 is technically radioactive, with a half-life considerably longer than the age of the universe, so...
Laser cooling wavelength: 425nm, but see below.
Doppler cooling limit: 120 μK.
Chemical classification: Transition metal, smack in the middle of the periodic table. Shiny.
Other properties of interest: Has a fairly large magnetic moment in its ground state, 6 Bohr magnetons, which means it has strong magnetic interactions. For this reason, it's kind of an interesting system to study-- you…
Element: Lithium (Li)
Atomic Number: 3
Mass: Two stable isotopes, masses 6 and 7 amu
Laser cooling wavelength: 671 nm
Doppler cooling limit: 140 μK.
Chemical classification: Alkali metal, column I in the periodic table. Yet another greyish metal. We're almost done with alkalis, I promise. Less reactive than any of the others, so the explosions in water aren't very impressive.
Other properties of interest: Lithium-7 is a boson, but has a negative scattering length, meaning that BEC's of lithium-7 tend to implode unless you modify the collisional properties. Lithium-6 is a fermion, and much…
Element: Francium (Fr)
Atomic Number: 87
Mass: Numerous isotopes ranging in mass from 199 amu to 232 amu, none of them stable. The only ones laser cooled are the five between 208 amu and 212 amu, plus the one at 221 amu.
Laser cooling wavelength: 718 nm
Doppler cooling limit: 182 μK.
Chemical classification: Alkali metal, column I in the periodic table. The heaviest known alkali, it's presumably metallic in appearance, if you could ever get enough of it together to look at.
Other properties of interest: Francium has no stable isotopes, and the longest lifetime of any of its isotopes is around…
Element: Strontium (Sr)
Atomic Number: 38
Mass: Four stable isotopes, ranging from 84 to 88 amu
Laser cooling wavelength: Two different transitions are used in the laser cooling of strontium: a blue line at 461 nm that's an ordinary sort of transition, and an exceptionally narrow "intercombination" line at 689 nm.
Doppler cooling limit: 770 μK for the blue transition, below a microkelvin for the red. The Doppler limit for the red line turns out not to be all that relevant, as other factors significantly alter the cooling process.
Chemical classification: Alkaline earth, column II of the…
Element: Xenon (Xe)
Atomic Number: 54
Mass: nine "stable" isotopes, masses from 124 to 136 amu. Xenon-136 is technically radioactive, but with a half-life of a hundred billion billion years, so, you know, it's pretty much stable.
Laser cooling wavelength: 882 nm
Doppler cooling limit: 120 μK
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with anything, so poses much less danger to scientists than any of the alkalis, though it has, at times, been used as an anesthetic, and Will Happer's group at Princeton has a funny story about a student's…
Element: Helium (He)
Atomic Number: 2
Mass: two stable isotopes, 3 and 4 amu.
Laser cooling wavelength: 1083 nm
Doppler cooling limit: 38 μK
(It should be noted, though, that despite the low temperature, laser-cooled helium has a relatively high velocity-- that Doppler limit corresponds to an average velocity that's just about the same as for sodium at 240 μK. This is because temperature is a measure of kinetic energy, and helium is much, much lighter than any of the other laser-cooled elements.)
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with…
Element: Rubidium (Rb)
Atomic Number: 37
Mass: two "stable" isotopes, 85 and 87 amu (rubidium-87 is technically radioactive, but it's half-life is 48 billion years, so it might as well be stable for atomic physics purposes.
Laser cooling wavelength: 780 nm
Doppler cooling limit: 140 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it’s a greyish metal at room temperature. Like the other alkalis, it’s highly reactive, and bursts into flame on contact with water, even more so than sodium (in general, the alkalis get more violently reactive…
Element: Sodium (Na)
Atomic Number: 11
Mass: one stable isotope, 23 amu
Laser cooling wavelength: 589 nm
Doppler cooling limit: 240 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it's a greyish metal at room temperature. Like the other alkalis, it's highly reactive, and bursts into flame on contact with water. For this reason, all physicists working with sodium have True Lab Stories about accidentally blowing stuff up with it.
Other properties of interest: Scattering length of around 80 a0; Feshbach resonance at around 900 G.
History:…
At the tail end of the cold-atom toolbox series, I joked about doing a "trading card" version shortening the posts to a more web-friendly length. In idly thinking about this, though, it occurred to me that if one were going to have cold-atom trading cards, it might make more sense to have them for the atoms, rather than the techniques. And having just devoted many thousands of words to technique, I don't really feel like trying to cut those down more, but atoms...
The "featured image" up top is a slide from my laser cooling lectures for our first-year seminar class. Elements outlined in red…
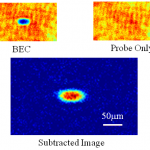
This is probably the last trip into the cold atom toolbox, unless I think of something else while I'm writing it. But don't make the mistake of assuming it's an afterthought-- far from it. In some ways, today's topic is the most important, because it covers the ways that we study the atoms once we have them trapped and cooled.
What do you mean? They're atoms, not Higgs bosons of something. You just... stick in a thermometer, or weigh them, or something... OK, actually, I have no idea. They're atoms, yes, but at ultra-low temperatures and in very small numbers. You can't bring them into…