Imagine a simple hike in a grassy part of South America. You hear a rattle and feel a quick stab of pain as fangs sink into your leg. Toxins in the snake venom travel through your blood vessels and penetrate your skin. If the snake is a South American rattlesnake, Crotalus terrific duressis, one of those toxins will be a phospholipase. Phospholipases attack cell and mitochondrial membranes destroying nerve and muscle function. Without quick treatment, a snakebite victim may be die or suffer permanent damage (1, 2).
The phospholipase from the South American rattlesnake is called crotoxin. Scientists are interested in studying crotoxin because snake bites are a serious health hazard and better treatments would help save lives and minimize nerve and muscle damage.
Today, we're going to explore crotoxin for a different reason. We're going to use crotoxin to investigate the four levels of protein structure. Crotoxin is a good protein for this activity because it contains both types of secondary structure, metals, disulfide bonds, and multiple protein chains. Some proteins don't work as well for this purpose because they are monomeric, or they only contain one type of secondary structure.
We'll use Molecule World for this activity, but other 3D modeling programs could also be used. Some people also use plastic tubes and magnets but if you have access to iPads, it makes sense to use actual structure models.
The structure we're going to use is 3R0L. If you touch the link on an iPad, the structure will be downloaded from the NCBI and you can open it in Molecule World.
Primary structure
The primary structure of a protein is the sequence of amino acids, joined in a chain, from the amino end to the carboxyl end. Crotoxin has four protein chains, each with a different sequence.
To view the amino acid sequences:
- Open the sequence viewer.
- Touch the atom icon and choose residues to see how the sequences in four chains are different.
- If you open the color key, you can see the colors and abbreviations for each amino acid.
 When residue coloring is used, each amino acid appears with a different color. We can see that these four amino acid sequences are all different.
When residue coloring is used, each amino acid appears with a different color. We can see that these four amino acid sequences are all different.
Secondary structure
The two kinds of secondary structure elements are alpha helices and beta sheets. These distinctive shapes are held together by interactions between atoms in the amino acid backbone.
To view both types of secondary structure:
- Touch the atom icon and choose Secondary view.
- Use the color key to identify both types in the structure.
A. Explore an alpha helix
To see how an alpha helix is held together:
- Find chain 3R0L-D in the sequence viewer.
- Touch the first set of green letters (LLQFNKMIKFE). One alpha helix will appear brighter.
- Open the Show/Hide menu and choose Hide unselected. The rest of the structure will dissappear.
- Touch the atom icon and pick the element coloring style.
- Open the Show/Hide menu and choose Complete backbone.
- Use the color key to identify the different atoms.
- Open the Show/Hide menu again and choose All atoms in residue. Now you can see where the side chains are positioned.
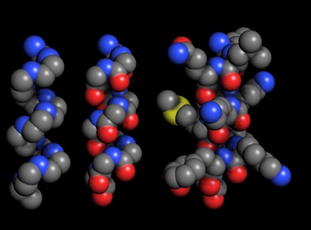 From left to right, this alpha helix shows the amino acid backbone, the backbone plus the oxygens, and the backbone plus the side chains.
From left to right, this alpha helix shows the amino acid backbone, the backbone plus the oxygens, and the backbone plus the side chains.
In an alpha helix, hydrogen bonds (invisible in this structure) form between the oxygens (red) and nitrogens (blue) in the backbone and hold the structure in a helical shape.
Touch the atom icon and Choose Reset View before going on to the beta sheets.
B. Explore beta sheets
To see how beta sheets are held together:
- Touch the atom icon and choose Secondary view.
- Scroll through the sequence viewer to find where the beta sheet begins.
- Touch all the letters in the beta sheet.
- Open the Show/Hide menu and choose Hide unselected.
- Touch the atom icon and pick element coloring style. The beta sheet looks like a loop.
- Open the Show/Hide menu and choose Complete backbone. This time, you can see the interactions between oxygens and nitrogens in different loops of the chain.
- Open the Show/Hide menu again and choose All atoms in residue to see where the side chains are located.
 From left to right, this beta sheet shows the amino acid backbone only, the backbone with oxygens, and the backbone plus the side chains.
From left to right, this beta sheet shows the amino acid backbone only, the backbone with oxygens, and the backbone plus the side chains.
Tertiary structure
The tertiary structure of a protein consists of a single amino acid chain plus any metals or prosthetic groups like heme or NAD.
Touch the atom icon and Choose Reset View before going on.
To see an example of tertiary structure:
- Touch the names 3R0L-D and 3R0L-other. The "other" row is a list of chemicals in the structure.
- Open the Show/Hide menu and choose Hide unselected. The objects that remain are a tertiary structure.
- Touch the atom icon and choose Secondary view. You can see this chain contains both types of secondary structure.
Explore bonds to metals
Touch the atom icon and Choose Reset View before going on.
- Touch the name 3R0L-D.
- Open the Show-Hide menu and choose Hide unselected.
- Touch the name 3R0L-D again to deselect it.
- Touch the Mn (manganese).
- Open the Selection menu and choose Select nearby.
- Open the Show/Hide menu and choose All atoms in residue.
- Touch the atom icon and choose the Ball and Stick drawing style and element coloring style. Now, you can see there are bonds holding the manganese atom in place. Similar bonds hold the sodium atoms in place as well.
Explore disulfide bonds
Touch the atom icon and Choose Reset View before going on.
- Touch the atom icon and choose Secondary View.
- Open the Selection menu and type C.
- Open the Show/Hide menu and choose All atoms in residue.
- Touch the atom icon and select the Ball and stick drawing style and element coloring style.
- Touch the names of chains A, B, and C twice to select and deselect them. Now, the cysteines will only be selected in chain D.
- Touch 3R0L-D to select chain D.
- Open the Show/Hide menu and choose Hide unselected. The disulfide bonds between chains can be seen.
Quaternary structure
The quaternary structure of a protein consists of the interactions between multiple chains.
Touch the atom icon and Choose Reset View before going on.
To view the quaternary structure of this enzyme:
- Touch the atom icon and the Spacefill drawing style and molecule coloring style.
- Open the Show/Hide menu and choose all atoms in residue. You can see all four chains.
One last fun thing we can do with this this protein is to identify the active site. In crotoxin, the active site is the place where this enzyme chops up phospholipids.
Exploring the active site
To explore the active site:
- Touch the atom icon and Choose Reset View before going on.
- Touch the name of chain 3R0L-D to select it.
- Open the Show/Hide menu and choose Hide unselected to hide chains A, B, and C. These chains are involved in binding to the cell membrane but they don't contribute to the phospholipase activity.
- Touch the atom icon and choose the hydrophobicity coloring style and the spacefill drawing style.
- Open the Show/Hide menu and choose Hide unselected.
- Open the Show/Hide menu again and choose All atoms in residue.
- Touch ACT in the 3R0L-other chain to highlight acetate. Acetate is a product of phospholipase activity.
- Hide the sequence viewer and turn the structure around to view the acetate inside the enzyme at the active site.
References
- Harris JB, Scott-Davey T. Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins (Basel). 2013 Dec 17;5(12):2533-71. doi: 10.3390/toxins5122533.
- Faure G, Xu H, Saul FA. Crystal structure of crotoxin reveals key residues involved in the stability and toxicity of this potent heterodimeric β-neurotoxin. J Mol Biol. 2011 Sep 16;412(2):176-91. doi: 10.1016/j.jmb.2011.07.027.





