
![]() In the late '90s, everyone started freaking out about their beef products, especially in Europe. Cows in Britain had started falling ill with a strange fatal neural disease. But not only the cows were getting sick - people who ate infected meat were hit too, dying by way of a new variant of Creutzfeldt-Jakob disease. The cow disease became known as Mad Cow for the way in which the infected cows acted. Soon enough it had become epidemic, and people looked for the cause - and found it to be a little protein, called a prion.
In the late '90s, everyone started freaking out about their beef products, especially in Europe. Cows in Britain had started falling ill with a strange fatal neural disease. But not only the cows were getting sick - people who ate infected meat were hit too, dying by way of a new variant of Creutzfeldt-Jakob disease. The cow disease became known as Mad Cow for the way in which the infected cows acted. Soon enough it had become epidemic, and people looked for the cause - and found it to be a little protein, called a prion.
Prions are misfolded proteins which cause diseases in animals. But beyond just causing disease in the individual with the genetic or environmental cause for the prion, prions are unique because they can infect healthy individuals like a virus. Somehow they cause normal proteins to misfold and mess up. Theories of how mad cow spread so virulently are varied, but most hinge on cows somehow eating the remains of other infected cows, thus taking in their proteins, including prions. The original source of the prion may have been sheep (whose remains might also have been fed to cows), who have a similar disease called scrapie, but the jury is still out.
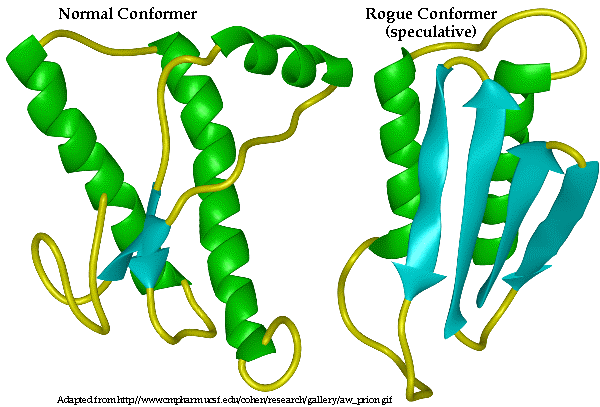 But how does a gene that encodes a protein that kills survive in a population? Most theories for how genetic disorders continue hinge on their usefulness in other scenarios. For example, the gene which causes sickle cell anemia, a debilitating and painful disease, is useful if you only carry one copy - you're healthy and you're malaria resistant. But what role do prions play which makes them evolutionarily useful?
But how does a gene that encodes a protein that kills survive in a population? Most theories for how genetic disorders continue hinge on their usefulness in other scenarios. For example, the gene which causes sickle cell anemia, a debilitating and painful disease, is useful if you only carry one copy - you're healthy and you're malaria resistant. But what role do prions play which makes them evolutionarily useful?
The part of the puzzle that had eluded scientists was what these prion proteins (PrPs) were doing in our bodies in the first place. We produce huge amounts of non-infective PrPs, but no one could figure out just why we do. Studies in mice were a dead end - mice who lacked PrPs seemed just as healthy as those who had them. Were PrPs useless? Perhaps evolutionary left overs? Scientists started to wonder. Then, some decided to try the same thing with fish.
Published in PLoS Biology, a new study finds that zebrafish lacking prions during development suffer terribly. Scientists infected fish embryos with morpholinos, DNA-like molecules that prevent PrP production. Infected embryos were unable to create the cell to cell connections which are vital to the development of internal systems. Simply put, the cells couldn't communicate to create the diverse differentiation of cell types for an adult fish. All of those that couldn't make PrP died.
So it turns out that the compounds which drive cows and people mad are incredibly useful. Just how they function in our systems isn't clear yet, but they probably have some role in cell to cell communication during development or differentiation.
This study provides the first clues into the uses and roles that PrPs play in the body. By learning what they do, scientists are one step closer to finding compounds that follow or disable misfolded PrPs that cause Mad Cow and Creutzfeldt-Jakob disease - and one step closer to finding ways to prevent them.
Edward Málaga-Trillo, Gonzalo P. Solis, Yvonne Schrock, Corinna Geiss, Lydia Luncz, Venus Thomanetz, Claudia A. O. Stuermer (2009). Regulation of Embryonic Cell Adhesion by the Prion Protein PLoS Biology, 7 (3) DOI: 10.1371/journal.pbio.1000055
