"A vacuum is a hell of a lot better than some of the stuff that nature replaces it with." -Tennessee Williams
Matter -- everything you know, love, hate, see, taste, and feel -- takes up space.

Even air must take up space. Not just wind, but still, stationary air takes up space. We've known this since Empedocles, in the 5th Century B.C., who had a clepsydra -- a hollow gourd with many holes in the bottom and a single hole at the top -- demonstrated it.

You plunge the gourd into a stream, lake or river, and it fills with water. If you lift the gourd up, the water leaks out of the bottom. But place your thumb or hand over the top, and the water will stop flowing out of the bottom. What prevents it from falling? It's got to be the air beneath the holes, exerting a pressure and taking up space!
You can even build one for yourself using (just like on Monday) a 2-liter bottle.

But why is this? Why -- at a fundamental level -- does air, or matter of any type, have to take up space at all?
Another way of asking this same question is, "why can't more than one object be in the same place at the same time?"

No matter how hard I try, I can't be in the same place at the same time as any other object in the Universe. And it isn't just me; I can't take any particle that makes up matter -- a molecule, an atom, even a proton, neutron, or electron -- and put an arbitrarily large number of them in a finite amount of space.

It didn't have to be that way! You can take photons -- particles of light -- and put an infinitely large number of them in an arbitrarily small space. Same deal with the 2nd, 3rd, and 4th heaviest fundamental particles in the Universe: the Higgs Boson, the Z-boson, and -- if you can overcome their charges -- even the W-bosons.
So why are protons, neutrons, and electrons -- the stuff that makes up all our normal matter in the Universe -- limited in this way?

It's the Pauli Exclusion Principle! Just like in baseball, where you can't have two runners on the same base, the Pauli Principle tells us that you can't have two identical fermions (one of the two basic types of particles, along with bosons) in the same state!
When I was younger, I used to think this was some minuscule technical detail of physics, good for little more than explaining the chemical properties of atoms due to their electron cloud structure.

Big deal, I can't put two identical fermions in the same quantum state.
But it's a much bigger deal than that. If I either cooled the temperature down to absolute zero or compressed matter with an arbitrarily large amount of force, I could squeeze any number of bosons into an arbitrarily small space.
But normal matter is made out of protons, neutrons and electrons, all of which are fermions. And this simple principle means that there's a finite volume that -- once it's occupied by one of these matter particles -- it's off-limits to the others!
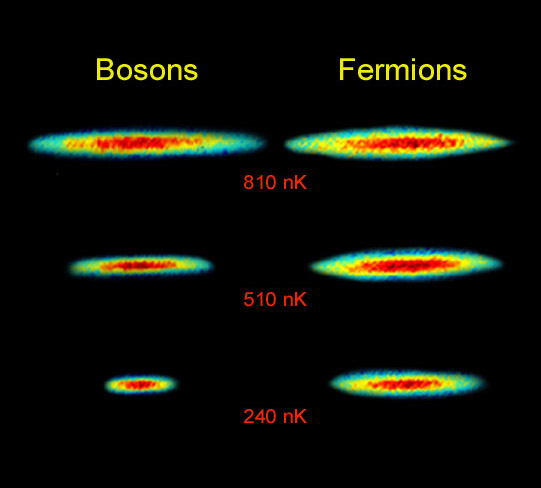
And that's why matter takes up space, no matter whether it's charged or neutral, and regardless of temperatures or pressures or any other physical properties!
There are some spectacular astrophysical consequences of this, and two of my favorites are what happens to stars when they die.

A white dwarf star -- somewhere around the mass of the Sun but the physical size of the Earth -- is made of plain old atoms, same as we are. But a white dwarf is about 300,000 times denser than we are! Yet, despite that incredible gravity compressing the white dwarf, the atoms refuse to buckle. Why?
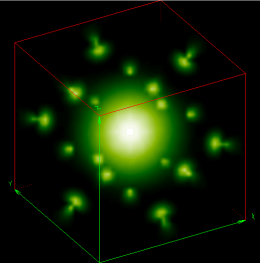
Because the atoms deep inside the star have their electrons bumping up against each other, and the electrons refuse to buckle and let other electrons any closer!
In fact, in the most extreme case, the electrons, rather than let another electron into the same state and violate the Pauli rule, would rather fuse with the protons, producing a neutron (and a neutrino), collapsing all the way down to a neutron star!

But neutrons are fermions, too, and even a star made entirely out of neutrons refuses to collapse! These are the densest known objects in the Universe that are still made of matter, and yet, they take up space!
So you can keep your bosons to yourself; my matter takes up space, and I've got the Pauli Exclusion Principle to thank for it! On the other hand, dark matter could be either bosonic or fermionic; we don't know yet, but my money's on bosonic, and that it truly doesn't take up any space! (How's that for some wonderment headed into the weekend?!)

Dear Ethan,
Matter does not occupy space. You may read my article
http://scienceforums.com/blog/444/entry-404-matter-does-not-occupy-spac…
After reading article you may ask question to me at blog or science forum.
From the blog:
"It says space is a existence and it is empty"
If your paper is as badly written as that, it fails already.
"Then, by present science knowledge everyone says FORCE is required to move a thing."
Nope, FORCE is required to accelerate a thing. Even then it has to be Net Force.
"An existence speed will be greater than zero"
More ill-presented wordage.
"Empty space has always zero average density."
Ah, Douglas Adams used this years before you in HHGTTG where the guide proves that there is no life in the universe.
Pretty much all your blog is exhaustively terrible. Learn to write. Of course I'm supposing that the problem is your writing rather than the thinking you're trying to express.
@Ethan - Why is your money on Bosonic? Or should I just be patient and wait for a future post to explain your position?
I've heard this explanation before, but I'm not sure I buy it for most of the normal matter we have here on earth. I've heard physicists much smarter than me say it, but I don't understand it. Certainly the Fermi Pressure is what holds up white dwarf stars, but I don't think it's the major thing for "normal density" matter.
Ferinstance, the H_2 molecule is bigger than an H atom, but that can't be because of Pauli exclusion between the two nuclei (since protons are spin-1/2, they can form a spin-antisymmetric state and have a symmetric spatial wavefunction) or between the two electrons (same reason).
The reason why the nuclei don't "overlap" in the hydrogen molecule is just electrostatics and "distinguishable-particle" quantum mechanics. Similarly, I don't think Pauli exclusion can be the only (or even major) thing preventing solid matter made of atoms (charged nuclei) from collapsing or having two things at the same place at the same time.
What am I missing here? Am I screwing up H2, or is there something about all other matter that's different than H2?
What happens in black holes? I thought that was matter that had collapsed on itself.
What happens in black holes?
What happens in black holes, stays in black holes.
[except for Hawking leakage.]
Anonymous Coward: It's not Fermi pressure per se, but that is the reason why there can only be two electrons in each orbit (one with spin +1/2, one with spin -1/2).
Were it not for the Pauli Exclusion Principle, all the electrons would happily drop down to the lowest-energy orbit, and the various elements would probably all look pretty much the same.
@Ethan - Great Post. But we still don't know why fermions cannot be in the same place. Is there any way to demonstrate the Pauli Exclusion Principle? Or anything more fundamental than that and not just descriptive?
Halfway through the story I started wondering: When did physicists start to distinguish between fermions and bosons?
The exclusion principle looks like a definition of fermions - but when did we start to call them that?
Great explanation, with good pictures. What's the tool that was used for the picture of the orbital wave functions?
Uh oh, this is entirely wrong. It is *not* the Pauli exclusion principle that keeps matter from collapsing on itself. It is the zero point energy, which applies to all particles both boson and fermions when they are confined in a small region.
Use the example of two protons in an orbit with a symmetric spatial wave function (e.g. s-wave). They have a binding energy, an attraction which holds them together. But the binding energy has the zero point energy to overcome. And to hold them infinitely close together would take an infinitely strong force. Consequently they remain a finite distance apart.
What about a Bose-Einstein condensate? All the particles are in the same quantum state. They can make those out of whole atoms nowadays. How does that relate to the Pauli Exclusion?
I'm no physicist, but my limited Science-Channel understanding of black holes is that all the mass was converted to energy and the energy used to warp space-time. The mass-energy is now stored in warped space-time itself. There is no actual mass or matter inside the black hole, so the Pauli Exclusion principle doesn't hold. Maybe?
Wow, what a long post to avoid answering the question. Saying that 'the Pauli Exclusion Principle' keeps two 'fermions' from occupying the same point in space is only a description of the phenomenon. It doesn't answer the question you asked, to wit: WHY does matter take up space? WHY are the physical laws governing matter the way they are? I'll give you a hint; you can find the answer in Genesis 1:1.
mad the swine: Really, you are going with a god-of-the-gaps answer here? At this level? We are talking about interactions between fundamental particles, and why they behave the way they do...and you posit it's because of some passage in Genesis? To be frank, everything we know, from particle physics to cosmology, is because we discarded the god of the gaps, and instead said, "no, really, why is this the way it is?"
Ugh, can't believe I'm going to say this, Ethan, but I agree with the creationist. Seems to me that saying the PEP is the reason things can't be in the same place is like saying Newton's laws are the reason there is gravity. Both strike me as more of a description of the phenomenon that fermions can't be in the same place. It doesn't seem like it gets to the deeper question, like relativity did for gravity. What gives?
Also @11 you are wrong. The answer can be found at www.venganza.org Seriously, it explains EVERYTHING!
I'm just a layman so excuse the post...
1) I read this as "matter takes up space because fermions take up space and matter is made of fermions"... Which doesn't really answer much. Why do fermions take up space?
2) I thought black holes (not neutron stars) were the densest things in the universe?
3) If bosons don't take up any space, how can they be said to exist?
RE #11 - "God done it" is not an answer, it's an assumption with zero explanatory power.
I'm with Ougaseon @13. The math seems like it's just a description of the physical phenomenon. Or is it the other way around, and at the quantum level the physical phenomenon is just an expression of the math?
I don't understand much (out of my league here)but I can answer part of the layman question in #14:
My undestanding of Black Holes is that the gravity and energy (gravity energy?) contained in a Black Hole is so great that it is another universe at the end of a black hole. All the laws of physics of this universe no longer apply when gravity is great enough to collapse matter into a singularity.
If I am wrong on this - I hope that some person with more knowledge will enlighten me. Please!
I think your description of the clepsydra by Empedocles has a bit of a twist here, the operation described in the Wiki states a gourd thrust into the water with top covered will not fill and that this is the justification for the premises of matter existing, though as you may be hinting, if you fill such container and cover the top, water will still run out the bottom⦠until an equalization occurs between the matter (water/air)left in the gourd and the barometric air pressure on the outside, given the state of mass properties of the gravity metric.
So from this lesson of late (490-430BC) we could state that normal matter seeks equilibrium and there are functions that can be described processes, such as the Pauli exclusion principleâ¦
There is a fundamental difference by which matter is defined in dark matter, and black hole matter, though even within the different states of different generations of matter, they should still be defined as seeking equalization within their generation.
I also strongly suspect the three generations of matter â¦Dark, Normal, Black
(not to be confused with the local generations of the standard model) also seek an equilibrium not only within but between their generations, while the energy of these forces always seek the lowest possible state, whether it be an atom or the universe as a whole, it is the dynamics of this attempted equilibrium that we call space. Time would therefore be lengthened by the expansion of the universe and the slow creation of ever denser forms of matter and would only be recognizable as we know it in the normal generation.
I suspect even black holes in a (hypothetical)pure vacuum would seek equilibrium by warping the distance between them.
And all of this leads one to *wonder* as you have so craftily done, Perhaps there is no such thing as identical fermions in cold dark matter and does the Pauli exclusion principle stand or is it warped? Does Gamma radiation resonance effect the field in which cold Dark matter resides?
It may be that the boson force is hidden by the Fermionâs configuration caused by a slight feeble feed back mechanism between black and dark matter, perhaps the large gamma radiation bubbles above and below the galactic plane serve in a Pregeometry dynamics of cold dark matter.
I would speculate Mass term hierarchy would not remain equivalent for these different generations of matter. D/N/B
Well thatâs how I see it, but then who am I?
My bet is that it still involves both constituents, Bosons and Fermions only the rules of engagement are not quite the same as with our local reality of normal matter.
There can only be one reason why matter occupies and curves space - matter IS curved space.
The idea is simple but realization not so much. But I believe we will get there one day.
What? No music for the weekend diversion,only wonder? I miss your picks.
Surely we can relate the PEP to some artistic medium.
I'll give it a shot...As one Fermion said to the other Fermion;
"Wish you were here".
copy/paste
youtube.com/watch?v=IXdNnw99-Ic&feature=related
Henk @7 asks: When did physicists start to distinguish between fermions and bosons?
Shortly after they discovered that particles have spin angular momentum in integer multiples of hbar/2. If the spin s (defined such that the spin angular momentum is hbar * s) is an integer, the particle is a boson; if s is a half-integer, the particle is a fermion. It was soon noticed that if you exchange two otherwise identical particles with arbitrary states, the two-particle wavefunction gets multiplied by (-1)^(2*s). The only way you can do this if the two fermions (for which the multiplier is -1) are in the same quantum state is for the wavefunction to be identically zero, which is the mathematical statement of the Pauli exclusion principle. No such restriction applies to bosons because the particle exchange has no net effect on the wavefunction.
In a universe in which fermions or their equivalent could be packed ever more densely, nothing would stop gravity compressing matter to a density incompatible with stars and planets as we understand them. That would also be a universe incompatible with life as we know it: a universe in which we would not be present to make observations.
These properties of matter are basically inescapable in a universe in which we could exist.
Thanks Eric Lund @21 for bringing this comment thread back into the realm of science. It was really going off the rails there! I was about to bring up the quantum field theoretic explanation for HOW the exclusion principle works, but I don't think I could've explained it as well.
Ethan has provided an excellent scientific explanation (science quibbling aside) of our familiar physical world.
There is always another question, a deeper mystery; that is a wonder of science.
The self referential aspect of language or mathematics means; that to understand a new insight, we must look with open eyes and mind at the wonder of our physical world (not just look at the words or math of a theory).
Religious myths, e.g. Genesis, generally make pretty lousy science theory; but most do not read religious texts to understand caterpillars or comets.
Re: Brian at #5
I agree that Pauli exclusion determines what the occupied ground state orbitals are, and is largely responsible for the chemical diversity of the elements.
But that's not at all the question Ethan is addressing here.
I agree with Ethan that for things like neutron stars and white dwarfs (dwarves?) that Fermi pressure (i.e. Pauli exclusion) is the dominant mechanism preventing collapse and answers the question as to why matter takes up space.
However, I still disagree that for "normal" charged matter on earth it's the dominant mechanism. The dominant mechanisms are electrostatics and what BillK calls "zero-point energy": the energy associated with localizing electron wavefunctions.
Pauli exclusion can't explain why the two nuclei in a hydrogen molecule don't collapse onto each other (see my above comment). My less-subtantiated claim is that, similarly, it's not the primary reason why my hand doesn't go through my desk.
Lieb's classic review and retrospective are relevant here.
What you've done (and what the Pauli Exclusion Principle does) is explain and describe HOW matter takes up space.
There is no explanation as to WHY it does so.
In an article on matter, you post a picture of a mountain and it's not the Matter-horn?! I'm beside myself with grief. In fact I'm so beside myself that I just accidentally poked myself in the eye. Excuse me for a minute will you?
Re: Ambitwistor at #26
I stand corrected on my "less-substantiated" claim. Thank you very much for the links!
Juice,
Fundamental physics doesn't answer "why" questions. That's the purview of philosophy (and it's questionable how well philosophy can "answer" such questions either). The job of physics is to describe phenomena in terms of laws, not explain why the laws themselves are true. If you ask physics "why does X happen", the answer is always "because X is predicted from the laws of physics, which themselves are supported by data Y".
See Feynman struggle to explain that physics can't answer questions like "why do magnets attract each other".
Or, perhaps one might say, physics answers "why" questions, but not in the way that laymen seem to expect.
Strictly speaking, matter for which the Pauli Exclusion Principle is the only thing keeping its constituents from coming together any closer is called degenerate matter. Most ordinary, everyday matter is not degenerate: other forces keep matter particles such as atoms from coming together too closely, e.g. electrical repulsion between electrons in atoms accounts for most typical matter taking up the space it does, and the fact that these electrons repel each other is the main reason why everyday solid objects are solid. Degenerate matter can only be formed under extreme pressures. Metallic hydrogen is one example, but it requires pressures greater by far than any achievable in any modern laboratory as of this writing.
The only form of dark matter we know of and can detect as of this writing is fermionic: the neutrinos. They're spin 1/2 just like most normal matter.
hi
@Wow, Now I have formulated the article. Your comments are appreciated. http://scienceforums.com/blog/444/entry-404-matter-does-not-occupy-spac…
that was fie rigth there .
I Think That The mass Is Diffrent
OK, it's now massively different. If it had been an improvement, this would have been a good thing...
Starting off, it goes into a valueless point. There being no consensus (its lack or measure has not been presented) does not indicate anything of import. Certainly not that your subsequent issues are indicated as plausible.
You make up a rule of nature next. Try one agreed by consensus. Failing that, one that is coherent and self contained enough to indicate whether your idea has any bearing on it.
Your next step is to insist that matter is 100% solid, when we know it's 99.99999999999999%+ empty space.
This isn't going to go well and it would require far more work on my part to correct you than you deserve on this second attempts' failure to consider whether you need to read up on the basics BEFORE making claims about science.