"It has been rightly said that nothing is unimportant, nothing powerless in the universe; a single atom can dissolve everything, and save everything! What terror! There lies the eternal distinction between good and evil." -Gerard De Nerval
(For Rich C. and Sili, for their questions on this post.)
The humble hydrogen atom -- one proton and one electron, bound together -- is the most common form of normal matter in the entire Universe. When you look out at all the galaxies in the Universe, what you're seeing is predominantly light coming from those simple hydrogen atoms fusing together at the cores of stars.
Yet despite the huge amount of hydrogen, something like 1080 atoms in our observable Universe, there's something far more abundant.

Light! If you want to understand where it comes from, we have to go back to the very early stages of the Big Bang. Not just back before there were atoms, but even before there were protons. The Universe was once so hot and dense that it was filled with what's known as a quark-gluon plasma.
When you heat matter up to temperatures above two trillion Kelvin (!), the fundamental components of nuclei -- quarks and gluons -- become so energetic that calling groups of them things like "protons" and "neutrons" doesn't make sense anymore.
Instead, quarks, gluons, and anything else that's energetically allowed, simply exists in an unbound state.
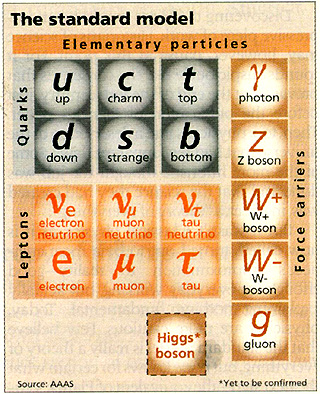
Well, the Early Universe had temperatures that put two trillion Kelvin to shame! And the hotter you turn up the furnace, the more stuff you make! In fact, in the earliest times, you not only made all the particles in the standard model...
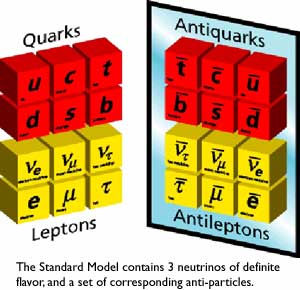
...you also made all the anti-particles in the standard model!
All told, in the very early stages of the Universe, what you think of today as radiation -- namely, photons, or particles of light -- was less than 2% of the total amount of particles present. (And if there's new physics at high energies, like supersymmetry, extra dimensions, or grand unification, it might be even less than that!)
But remember the most important property of the Universe in the cosmological standard model: it's expanding and cooling as it ages.

One result of this is that less and less energy is available, so it gets harder and harder to make these heavy particles. Another result is that they're progressively farther apart, meaning that they start to condense into bound objects: baryons and mesons. And finally, because the Universe is aging all this time, the unstable particles that you've made decay!
Some of the particles that decay are (likely) responsible for creating the matter/antimatter asymmetry in our Universe, but the vast majority decay into truly stable radiation: photons and neutrinos.
The net result is that our Universe is filled with something like 1080 protons (and electrons), but something like 1090 photons. In other words, we have over a billion photons for every "atom" we're going to wind up with. For many thousands of years, protons and electrons combine to form neutral atoms. But every time they do, an energetic enough photon comes along in very short order and kicks the electron out, keeping us in a plasma-like state. So we wait.
The Universe continues to expand and cool, and will do so until it's cool enough to form neutral atoms for the first time.
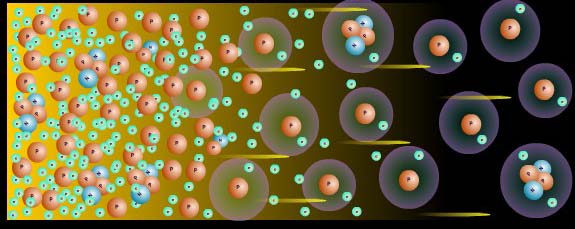
What determines when this happens? Well, remember how I said that photons outnumber atoms by more than a billion to one? That means that not only does the average photon energy need to be unable to ionize a hydrogen atom, but that less than one out of every billion photons needs to have enough energy to ionize it.
So that very tail-end of the spectrum needs to drop below the ionization energy/temperature of hydrogen. This happens when the Universe cools to a temperature of about 3,000 Kelvin, almost two orders of magnitude lower than what it would need to cool to if the number of atoms and photons are the same.
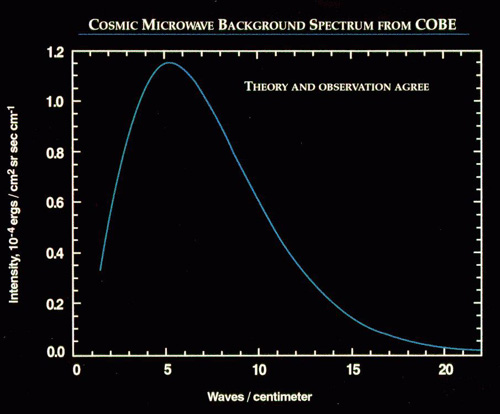
When you finally cool down enough to form neutral atoms -- when the Universe is about 380,000 years old -- that radiation left over from the Big Bang can finally travel to you in a straight line, cooling as the Universe expands, and reaching our satellites and telescopes today, 13.7 billion years later.
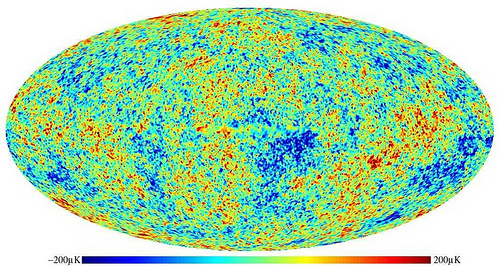
On average, we see that leftover radiation at 2.725 Kelvin, but when we look at even finer resolution, we can see what the fluctuations are in that radiation! That's what this famous WMAP image shows us: how much hotter or cooler each individual place in the sky is from its "average" temperature of 2.725 Kelvin.
Now that you know what causes it and where it comes from, we can ask the big questions: what does this sky map mean, and what do we learn from it?
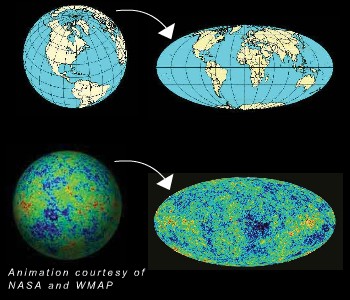
First off, this ellipse shows the entire sky. The plane of the galaxy has been subtracted out to the best of our abilities, and the minimum resolution of this image is between about 0.1 and 0.2 degrees, limited only by the sensitivity of our instruments.
What we then do is look at different sections of this map. For example, we might break it up into four equal chunks, and look at what the average temperature is in each of those four chunks, and see how far they deviate from the average. We might then try again, except this time, we break it up into twice as many chunks. And we do it again and again, seeing how the magnitude of the average fluctuations change as our scale changes.
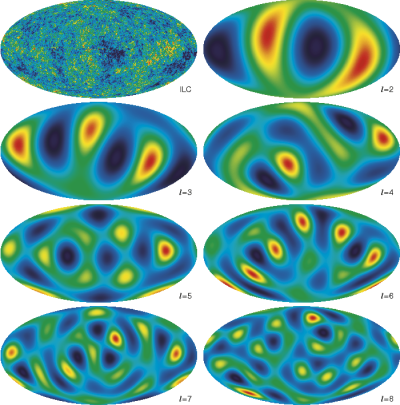
And we do this up to the maximum resolution of our images, measuring what the average temperature fluctuations are on a whole slew of different scales. And, as we like, we can make a graph of what this looks like!

So when you see this graph, that's what we're looking at! For instance, the maximum average temperature fluctuations -- of around 70-80 micro Kelvins -- happen on scales of just about one square degree.
This is incredibly interesting for learning about the Universe! Why? Because, according to our leading theories, the Universe began with equal fluctuations on all scales! But by time the Universe is 380,000 years old, that is very clearly not the case anymore. Why not?
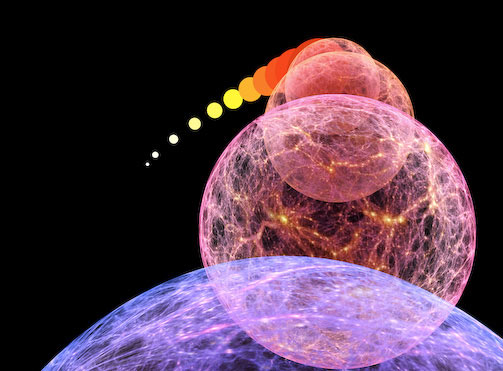
Because structure has started to form! The slightly overdense regions have started to grow; the slightly underdense regions have started to shrink. Radiation pressure has pushed small collapsing regions back outwards (in some small-enough regions, multiple times), but hasn't had time to push large enough ones outwards even once!
In short, the patterns that come out of these fluctuations are very sensitive to the amount and type of matter and radiation both in the Universe at that time -- when it's 380,000 years old -- and also how it's been expanding since.
So when we analyze this data, we can learn how much matter (and of what different variety) exists in the Universe at any given time!

And that's what the WMAP image means, where it comes from, and what we use it for!



I think I just lost the Starts With A Bang drinking game, if possible.
Good post, though. I wouldn't mind a refresher, or just a reminder if Ethan addressed it before (and I seem to remember that he did), on how we can derive the percentages of dark matter/dark energy/interactive matter from the WMAP data. And does that assume that dark energy functions as a cosmological constant?
Has fractal decompression/clarification of the WMAP image been used?
Ohhhhhhh!
*slaps forehead dramatically*
Thank you!
I'm not sure I understand this, though. I thought the fluctuations were down to Heisenberg uncertainties. Why should there be 'big' ones? Or shouldn't there at least be less big ones according to some sort of Boltzmann distribution?
Sili,
There is a Gaussian distribution in terms of the magnitude of fluctuations on any one particular scale. What's plotted above in the fourth-to-last image is the average (ok, technically, RMS) fluctuation magnitude on a given scale. The fluctuations, as you correctly deduce, are due to quantum uncertainties. However, because of inflation and the exponential expansion of the Universe that sets up the Big Bang, these fluctuations get stretched across all scales, so that we wind up with an initially scale-invariant spectrum of fluctuations.
This last bit is extremely important for structure formation; we are sensitive enough to detect that the spectral index to form the right amount of structure on all scales must be extremely close to 1, and it could have been anything from -1 to 3.
In models of slow-roll inflation, such as New Inflation (the simplest set of working models), the spectral index is extremely close to -- but not exactly equal to -- one. The simplest working models predicting a value somewhere between 0.92 and 0.98. Our best observations, AFAIK, give us that n_s = 0.97 +/- 0.02. The index rolls by less than 0.1% between the largest and smallest observable scales in all such models.
(If you remember back to Monday's post, that rolling index of a factor of something like 1,000 is what's required to form primordial black holes. Hence, my doubting of their existence.)
"that less than one out of every billion photons needs to have enough energy to ionize it"
If photons and electrons/protons were equally bumping into each other, you could argue this way, but photons do not bump into each other and they travel at light velocity while the electron trying to bump into a proton is relatively slow at 3000K.
Those 10^90 3.7 degree K photons have a mass of about 10^21 solar masses, or about 9% of the mass of the observable universe. I wonder how much mass the no longer massless neutrinos have?
Geoff @2,
The WMAP image that we display does not have the galactic mask in place, and is not trustworthy for doing science with. You can download the original files from the WMAP/LAMBDA site, but I do not know what sort of image processing has been done to the visually striking map that I always show here, save to say that it is severe.
Africangenesis @6,
Your math is off by quite a bit. Those photons have a total energy of something like 0.01% of the total energy of the Universe. Neutrinos, because they have a mass, come in at a bit more: somewhere like 0.1% of the Universe's mass. That may not seem like a lot, but it's perhaps comparable to the amount of mass that the Universe has in the form of planets.
Sascha @5,
Although you're correct that interaction rates typically run at ~ number density * cross-section * velocity, and the velocities of the two processes are very different, when we talk about emission of the CMB we are talking about the surface of *last* scattering. You must have *all* of the atoms become neutral forever, a process that takes more than 100,000 years to complete. (Remember that the number of high-energy photons you have goes *up* as the number of ionized hydrogen atoms goes down.) If you form neutral hydrogen, you typically emit a Lyman-series photon, which will eventually run into another hydrogen atom, ionizing it (for all intents and purposes). Redshifting the Lyman-series photon to an unabsorbable wavelength is rare; given the high densities, the interaction rate is still very high. You need the very rare two-photon-emission to occur, where the back-reaction rate is negligible. (Peebles has a good discussion of this.)
In summary, your logic is reasonable, but based on the incorrect assumption that the reaction and back-reaction rates are independent, and that once one exceeds the other everything becomes neutral.
Science, it works!
http://xkcd.com/54/
Dear Doctor Siegel,
Thank you for who you are and what you do.
I am unspeakably grateful for your writings here and have waited far too long to express how much you nourish and sustain a young mad scientist within me.
An elderly and retired computer engineer now, I remember with vast fondness the budding me in fifth grade reading about the exploits of The Mad Scientists' Club and my longings to be among them and involved in their calculated and considered endeavors.
I would likely have traded my younger brother for the Internet we have now, and Wikipedia, and this computer which holds all my photos and movies and music and writings and connects me to a metaphorical "cosmos" of libraries and academic institutions. I love watching the lectures at MIT. I love reading Philosophy at Stanford, humor at XKCD and Abstruse Goose, and about the origin of words and phrases at World Wide Words.
Did you know that the phrase "Dog Days" is based on the prevalent constellation of hot summer, Sirius? I just thought it was because dogs hang in the shade at that time. Only mad dogs and Englishmen go out in the midday sun (that's a tune lyric, not a phrase from a poem as I was taught).
I live in the mountains and have a border collie now who accompanies me in every moment. We're outdoor creatures and there is little light pollution here and we love long walks through the woods in the moonlight. But on clear nights, moonless nights, we linger together in the gazebo and stare at the endless heavens and consider the reaches of the Cosmos. Every month I print out a current Star Chart to garner some understanding of what I'm seeing.
You're one of those Elders who drips the magic and poetry and Truth of it in every utterance. Not "more" than we know. Just "what" we know.
You're one of those Mentors who shows a way of being that embraces logic and love and delight in every posting. You're one of those Scientists at the edge of the universe squishing back the uncertainty with every experiment. It really is very gratifying to know how an LED works. Those little electrons are jumping in their orbits and collapsing with photons. And you can see them!
I so love your obvious delight in this.
Hydrogen is burning. Burning I say! Burning! The very thought of it -- atoms on fire -- makes my head vibrate.
We figured this out. We're just naked apes pushed all bloody and gore-covered from a mother's womb, helpless and hungry and screaming and terrified of a world we did not envision and don't understand.
And yet we figured this out.
We make steel, polish lenses, measure the speed of light (wow!), build equations for refraction and convergence, posit ideas and test them, slam parts of atoms into each other, and make a map of the universe.
You can add waves from telescopes on opposing sides of the solar system. That is so cool!
Thank you, Doctor Siegel, for consistently making me feel as no other writer I know does, like a standing member in the Mad Scientists' Club who revels in the magic and mystery of the lovingly revealed and deeply honored universe.
Did I remember to say thank you for who you are and what you do.
Oh, yes. It bears repeating. Thank you.
Dr. Siegal,
Apologies, I used the wrong wavelength for the photons at 3.7K but, when corrected to 1.9mm it still comes out larger than your calculation, I enter these in succession to wolframalpha.com:
energy of 1.9 mm photon
(1.045Ã10^-22 joules) times 10^90
1.045Ã10^68 joules/(c^2)
1.163Ã10^51 kilograms in solar masses
5.849Ã10^20 solar masses
which is about 3.9 pct the mass of the universe
I don't know how good an estimate wolfram has for the mass of the universe, but I also don't know how good your 10^90 figure is.
Do we have any idea how many neutrinos there are? Do we know what pct the speed of light they travel? Is there literature on this? -- thanx
Africangenesis,
Stars make up only about 0.5% of the total energy of the Universe, not 100% as you assume. You need to scale down your photon estimate by a factor of 200 to reflect this. And when you do, you will notice that you come within a factor of 2 from the number I gave in the post.
You will also note that there are some simplifying assumptions: 10^90 is quite a rough figure, you did not integrate over the photon energy distribution, instead treating "average energy" as a substitute for that, and the "average" CMB temperature is 2.7 K, not 3.7 K. Still the numbers come out much better now, don't they?
We know all about neutrinos from the Big Bang as well, including having some data from neutrino oscillations from their rest mass; the literature is so extensive that you can find a huge deal of the information by reading the wikipedia page on "neutrinos."
Ethan
I must admit that I need a little more explanation.
Consider the three paragraphs where you say, "So that very tail-end of the spectrum needs to drop below the ionization energy/temperature of hydrogen. This happens when the Universe cools to a temperature of about 3,000 Kelvin... when the Universe is about 380,000 years old.... On average, we see that leftover radiation at 2.725 Kelvin."
I sort of follow via you, Wiki and Weinberg (e.g Weinberg pg 528-529 blackbody radiation formula 15.6.1 but (G) between (F) and (H) are confusing.)
? ? ? What I don't understand is how at 380,000 years the 3000 K Hydrogen ionization temperature relates to the then temperature of the universe which, via Weinberg (H), seems to be at least 10^30K? ? ?
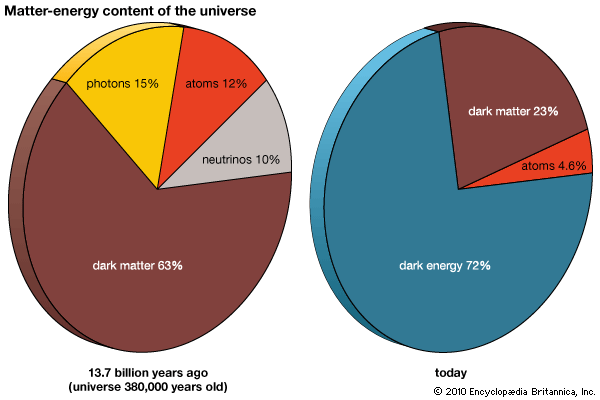
Also, in the second to last chart, I assume that the two pie charts are NOT drawn to scale; thus NOT giving the relative absolute Matter-energy content of the universe at 380,000 years versus now.
? ? ? So in the standard big bang model cosmology, has there been conservation of Mass/Energy from 380,000 years ABG to now? or not? ? ?
I thought the standard big bang theory says NOT; but the two pie charts suggests YES. So please help me understand. Thank you for any clarification.
Thanks! This is very helpful, and I appreciate you taking the time to explain it.
OKThen,
You have misunderstood Weinberg. The temperature of the Universe today is 2.725 Kelvin. The temperature at some earlier time -- or at some higher redshift, z -- is equal to the temperature today (T_0) times (1 + z). The Universe at age 380,000 years corresponds to a redshift of about z=1088, so that's where I get 3,000 degrees from. You have to extrapolate back to nearly the Planck energy to get a Universe of 10^30 Kelvin.
As the Universe expands and ages, the energy density goes down. But if the Universe were to re-contract to become the size it was at 380,000 years once again, the energy-density pie chart would again go back to what it was back then. It isn't energy explicitly that's conserved, but there are many symmetries and conserved quantities.
I don't understand this logic.
Temperature is relative between 2 or more objects, it is not an absolute. The early universe was only hot compared to TODAY.
There is only a photon exchange between particles if one of the objects has a higher temperature.
Since temperature differences where not nearly as great in the early universe, why would there be more photons?
Nowadays absolute zero, the hypothetical but unattainable temperature at which matter exhibits zero entropy, is defined as being precisely 0 K and â273.15 °C.
Those days zero entropy was attainable at a much higher temperature.
Ergo, there should have been less photons compared to now, not more.
Ethan
I did misunderstand. I sort of knew what you just explained; but not well enough to put my finger on the rut I was digging myself into. And the more I tried figure it out; well I just got more confused. So, thank you for quickly clarifying.
#9 ehj2
And your thoughts are well appreciated too. Well said.
wolframalpha.com must have either their mass of the universe or their black hole calculations wrong:
mass of the universe
gives 3x10^55 grams
multiple that by 3:
black hole of 9x10^55 grams
gives an event horizon radius of 1.337x10^26 meters which is 14.13 billion light years
If the observable universe were that close to being a black hole, I think someone would have figured it out by now.
"Since temperature differences where not nearly as great in the early universe, why would there be more photons?"
Having two photons of equal energy pass each other and absorbed in the emitter of the other photon means there are TWO photons, NO heat exchange.
No heat exchange != No photons.
Is the graph (some integral of) the Fourier transform of the background data?
@wow:
you think of an exchange of photons between objects with the same temperature. What one object is loosing on energy it regains by the other. Then a big object with a larger surface would loose more photons than a small object. You would be able to create entropy just by putting them together in an isolated environment and wait.
WMAP can see photons only when cooled to a temperature BELOW the radiation from the Big Bang. At the same temperature it would not detect anything.
... keeping us in a plasma-like state
Ethan, what does "plasma-like" mean, here, and how does it differ from plasma? I'm assuming the "-like" indicates you being careful about distinctions.
Looking closer at that moment of transition to neutral hydrogen... over the next N (1e5?) years we experience a phase transition, with a nearly constant average temperature, right? Every time we cool, infinitesimally, a few ions bind and release enough energy to heat us back up.
This can't happen uniformly, though, can it? There will be hotter and cooler bits. The electrons respond to field gradients a thousand times as fast as the protons, scattering off photons that hardly bother the protons bumbling about, trying to keep up. It seems very unstable and non-linear, tending to amplify fluctuations, and stays more or less just that way (with just a slowly rising fraction of neutral bystanders) for quite a long time, as birth-of-the-universe durations go. The hiatus seems to allow for evolving quite a lot of structure that will be frozen in place when the phase transition completes and interaction cross-sections collapse.
Is there any reason beyond mathematical convenience to think that all these nonlinear interactions fail to leave us with anything more complicated than a cooling cloud of ideal gas, and with only the non-uniformities left over from inflated quantum fluctuations, faithfully preserved?
"you think of an exchange of photons between objects with the same temperature"
Yes· This is correct.
"Then a big object with a larger surface would loose more photons than a small object"
And it receives more photons than a small object.
"WMAP can see photons only when cooled to a temperature BELOW the radiation from the Big Bang"
No, it can see them at a temperature ABOVE the radiation from the big bang. However it also sees the radiation from itself. This is the "Noise". Reduce the temperature, reduce the noise. Then your signal becomes easier to retrieve from the noise.
You seem to be SEVERELY confused about radiation and detectors.
@wow
You are right, with a lot of 'noise' it is possible for WMAP to detect at a higher temperature. You can filter out the (higher energy) 'noise' if not too dominant I suppose.
In your model you confirmed there is a replacement of photons with equal energy possible so there will be no net change in temperature.
Would it not be logical in your model to assume that in the case of a cold and a warm body there would also be an exchange of photons too? The "cold" photons replace the "warm" ones on the hotter object and vice versa.
This would mean that "cold can flow to warm if warm flows to cold".
There is light because the sun has a higher temperature. A lightbulb in a room without windows produces visible photons. This is only possible because of a much higher temperature of the sun or lightbulb in respect to the surroundings.
In your case - you are trying to make light (photons) with something that has the same temperature as the rest of the room. How do you do that?
"You can filter out the (higher energy) 'noise' if not too dominant I suppose."
Unfortunately it's random.
That means you don't know what signal you should have and therefore cannot just subtract the mean of the noise.
This is one of the reasons why you cannot get a climatological temperature trend from less than 30 years worth of data (in our current state, you can often get away with 20 years), because the noise being higher than the signal at 10-15 years means you cannot extract the signal unless you know what the noise did (which being random isn't possible).
"Would it not be logical in your model to assume that in the case of a cold and a warm body there would also be an exchange of photons too?"
Yes.
"The "cold" photons replace the "warm" ones on the hotter object and vice versa."
The only difference between the "cold" photons and the "warm" ones is that there are more warm ones: blackbody radiation curve: higher temperature body has much more energy leaving it as photons.
"This would mean that "cold can flow to warm if warm flows to cold"."
No, photons move from body to body. Even a cold body has photons leaving it.
"There is light because the sun has a higher temperature."
There is VISIBLE light from the sun because it has a higher temperature. Wein's Displacement Law.
"A lightbulb in a room without windows produces visible photons. This is only possible because of a much higher temperature of the sun or lightbulb in respect to the surroundings."
Indeed.
And, if you were able to stand on the sun and point an especially effective telescope at the earth to zoom in on that lightbulb, you'd be able to see that lightbulb: it's photons are going to the sun.
"In your case - you are trying to make light (photons) with something that has the same temperature as the rest of the room."
Yes. Remember you get photons from a body at room temperature. Just get an IR camera to check.
"How do you do that?"
How do you stop it?
"A lightbulb in a room without windows produces visible photons. This is only possible because of a much higher temperature of the sun or lightbulb in respect to the surroundings."
Oh, what IS wrong there is the "of the sun" bit.
http://en.wikipedia.org/wiki/Flame
A candle gives off light even if the windows are open and despite the sun being ~6,000C whilst the flame is around 1,000C.
You would have a candle emit no light in an open room depending on whether you had the window open or closed.
And for incandescent light bulbs, they remain below the 6,000C of the sun:
"Tungsten filaments radiate mostly infrared radiation at temperatures where they remain solid (below 3683 kelvins / 3410 °C / 6,170 °F)"
From the wiki page on incandescent light bulbs.
Does the light go out when you open the window, Hannes?
"You are right, with a lot of 'noise' it is possible for WMAP to detect at a higher temperature."
For further information on this: the dark current noise of a CCD increases by twofold for each 7 degrees C of temperature.
THAT is the "noise" you reduce by cooling your detector.
When you're only getting a few photons, the spurious signal caused by the temperature of the device moving electrons into the electron well is a big problem.
But the photons leaving the 3Kelvin background can be detected by a radio antenna on the earth at 300Kelvin. The radio wavelength photon isn't "repelled" by the heat of the radio antenna.
@wow
You are sincere in your answers, but I do not now if you understand my concern here.
"Would it not be logical in your model to assume that in the case of a cold and a warm body there would also be an exchange of photons too?"
"Yes."
- This would mean that "cold can flow to warm if warm flows to cold".
"NO, photons move from body to body. Even a cold body has photons leaving it."
- Why NO? So a cold body replaces the hotter photons on the hot object, yes or no? Please, your answer is not clear to me.
The question is in my case is WHY a photon is produced in the first place on a cold body when there are for example only hotter objects in its surroundings?
An IR camera is not different from a normal camera or your own eyes. It can only "see" objects when they have different temperatures. When there is no difference in temperature what would it see?
Is temperature absolute or is it relative in your opinion?
"- Why NO? So a cold body replaces the hotter photons on the hot object, yes or no? Please, your answer is not clear to me."
I don't understand your concern here because your concern is incoherent mumbo-jumbo.
A cold body above absolute zero emits photons. These photons are emitted, even if there's an entire universe of hotter bodies around it.
"The question is in my case is WHY a photon is produced in the first place on a cold body when there are for example only hotter objects in its surroundings? "
The existence of hotter bodies doesn't stop emission of any other body, hot or not.
The answer to your question is that a cold body produces photons the same way hot bodies produce photons.
"An IR camera is not different from a normal camera or your own eyes. It can only "see" objects when they have different temperatures."
Again, this is complete nonsense.
An IR camera, like our eyes, captures photons and registers their effect on the sensory organs.
Temperature has NOTHING to do with it.
A photon is emitted from a body.
A sensor detects that photon when it absorbs the photon.
The hotter a body, the more photons it will produce and the higher the maximum photon energy emitted will be.
Tell me, does your lightbulb go out when you open the window?
"So a cold body replaces the hotter photons on the hot object, yes or no?"
No.
(remember: YOU demanded a solely yes or no answer)
"radiation left over from the Big Bang can finally travel to you in a straight line" - And how would any kind of straight line manage to traverse vast quantities of space-time between two given points (say, a star and your eyeball) which are both in constant independent and relative motion while also reportedly suffering from the vast effects of dark matter and energy in addition to the continuing space expansion? I'd need that explained in terms of expanding wavefront incidents or something more simple,perhaps.
@Wow
"A cold body above absolute zero emits photons".
Relative to what?
You believe in an absolute temperature, according to your answer.
I try to explain that temperature, even at the lowest possible temperature is time-dependant. Thus a relative measurement, depending on the observer.
"Relative to what?"
No relative to anything.
If a water sprinkler emits water, then it emits water. No "relative to what". It emits water.
If a lightbulb emits light, then it emits light. No "relative to what". It emits light.
If a loudspeaker emits sound, then it emits sound. No "relative to what". It emits sound.
"You believe in an absolute temperature, according to your answer."
Do you know what "absolute zero" means?
"I try to explain that temperature, even at the lowest possible temperature is time-dependant."
Nope. The units of temperature are "kelvin" or "Celsius" or "Farenheit".
No "per second" there.
Go look at this page:
http://en.wikipedia.org/wiki/Units_of_measurement
Temperature isn't time dependant.
You also haven't "explained" it, you merely stated it.
"Thus a relative measurement, depending on the observer."
And the observer doesn't make it time dependent either. The weight of a 1 Kg mass doesn't change because you're looking at it.
Here's an experiment for you. A thought one to make the maths easy.
We have one cold body enclosed in a hotter body and that body is enclosed in a perfectly insulating container, so no energy is lost from the system.
The inner object emits photons and loses energy by radiative losses.
e=sigma t^4
The outer body emits photons and loses energy by radiative losses.
E=sigma T^4
The hotter body loses MORE energy from its photons than the colder body because T is greater than t, ergo E is greater than e.
With me so far?
Now, what rate does the hotter body cool?
It loses E watts of thermal energy. The colder body gives it e watts of thermal energy. Therefore the energy loss from the hotter body is:
E-e = sigma (T^4 - t^4)
If under YOUR hypothesis, the colder body DID NOT have its photons absorbed by the hotter body, then the energy loss of the hotter body would be:
E = sigma T^4
This is incorrect.
Additionally, since your hypothesis allows the colder body to absorb the photons from the hotter body but not emit any photons, it warms by
E = sigma T^4
Rather than the (correct)
E - e = sigma (T^4 - t^4)
(since E > e and we're calculating the WARMING of the cooler body)
"And how would any kind of straight line manage to traverse vast quantities of space-time between two given points..."
If the two points moved differently, then it would be a different photon that managed the path between both points. However, it would still be a photon from THAT star to YOUR eye. Just not the one that would have done that specific trip if you hadn't moved your head to the side.
----.
Ethan, if you are with "Wow" I am completely disappointed.
So you don't know or at least can't respond to the equations in post 34.
You can't because you've been misled and WILLFULLY accepted the lies you've been regurgitating here.
You're completely disappointed that you cannot explain your woo-like theories.
I am disappointed that someone with a professional career does not understand some basics here.
"Work" can only be created in a thermodynamical way if there is a relative difference in temperature.
Two trillion Kelvin temperature does not say anything, because it is relative.
Uncooled detectors use sensors that work by the change of resistance, voltage or current when heated by infrared radiation. These changes are then measured and compared to the values at the operating temperature of the sensor.
If in a isolated room there are no differences in temperature (= even small ones) an uncooled IR detector will see nothing.
It is as blind as a mole. Zero detection of anything. Not even a photon.
@Wow
"If under YOUR hypothesis, the colder body DID NOT have its photons absorbed by the hotter body, then the energy loss of the hotter body would be:
E = sigma T^4
This is incorrect."
-You look at the early universe 13 billion lightyear away in the PAST.
How is it possible that the hotter object (= the early universe) receives the "COOLER" photons from us?
The photons would have to move to the past. Think again.
> How is it possible that the hotter object (= the early universe) receives the "COOLER" photons from us?
Because of redshit, dunce.
NOTE: This has nothing to do with your earlier intent that cooler bodies don't radiate. E.g. our earth is warmer than the microwave background, yet the universe is still radiating at us.
"The photons would have to move to the past."
No, they wouldn't. They'd have to be radiating from a body that is moving away from us very fast.
I also note that you haven't explained why your hypothesis doesn't get the radiation of a black body incorrect. Instead you galloped off into the sunset and insisted that photons would have to be cooled somehow.
@wow
An object from the past which had a trillion °C compared to us now.
Still it is radiating almost at â273.15 °C.
It radiates the same wavelength as an artificially cooled object nearby with the same temperature (a BEC for example).
This initially very hot object, will it cool down or heat up any observer in the present?
"An object from the past which had a trillion °C compared to us now."
Yup.
Which means that a photon redshifted from the hard-X-Ray spectrum is now in the IR range.
"Still it is radiating almost at â273.15 °C."
Yup, where the peak is in accord with an extreme redshift above.
"This initially very hot object, will it cool down or heat up any observer in the present?"
What does this have to do with the spectrum of the body? NOTHING.
Without that 2.7k background radiation, the earth and other bodies would be cooler than they are without it. Therefore the background radiation warms all bodies within the universe.
@ WoW
Finally we do agree above some above.
So is cool and warm relative or not?
So where within this early universe with a trillion °C is an "absolute" lowest temperature of â273.15 °C feasable?
What determines your "absolute" minus temperature? Is it time dependent or not?
-I don't think â273.15 °C as a lowest measurable temperature was an option in the early days of the universe...
"So is cool and warm relative or not?"
ALL temperature is relative. It is ALSO relative to absolute zero, the coldest anything can possibly be.
"So where within this early universe with a trillion °C is an "absolute" lowest temperature of â273.15 °C feasable?"
Nowhere.
"What determines your "absolute" minus temperature?"
Go look up in ANY book on thermodynamics.
Energy, expressed as random stochastic motion within a lattice or molecule's motion within a volume.
"Is it time dependent or not?"
Not.
"-I don't think â273.15 °C as a lowest measurable temperature was an option in the early days of the universe..."
Just because an F1 driver can't go in reverse on an F1 race (is not an option) doesn't mean there's no reverse.
You are SERIOUSLY deluded, by the way.
Here is your absolute error in all things temperature for you put down as a question you can answer and even test yourself.
First the situation as science knows it to exist:
Bodies at any temperature above zero absolute radiates temperature. The total energy of that radiation depends on the temperature of that body to the fourth power.
ANY body will do so.
The situation in a scenario easy to calculate:
One body is at 10x the temperature of another, both in a vacuum. If the hotter body radiates 100W of heat radiantly, the cooler body will lose 0.1W of heat radiantly.
If the heat only transfers between these two bodies, the warmer body loses net 99.9W of heat. Therefore after a time it is warmer than it would be if the cooler body wasn't there.
Now the situation in a test of the laws of thermodynamics, with an answer you can give:
If you have a body that is at a stable temperature with 99.9W of energy pumped into it in a chamber at 1/10th the temperature of the hot body. If you place that body inside a large chamber that is 1/5th the temperature of the hot body, that body is now losing 100W of energy in radiation from the body, but the chamber is radiating 0.8W.
This means that the hotter body, despite being in a cavity that is cooler than itself, is losing a net energy of 99.2W whilst 99.9W is being pumped in. A net increase in energy input into the system of 0.7W.
Does this hot body heat up in the cool cavity?
Well?
Does the cool cavity warm the hot body or not?
@Wow
In the early universe you want to maintain â273.15 °C as absolute zero?
Thermodynamics during that age determined the lowest achievable possible temperature, not you.
I call that thermodynamically lowest possible temperature "absolute zero".
If the universe expanded this "absolute zero" changes with it.
Do you really think this epoch where we live in is so special, that we can only now come so close to "absolute zero"?
I already answered your earlier question about warm and cool being relative.
"In the early universe you want to maintain â273.15 °C as absolute zero?"
It's got nothing to do with what I want. It is absolute zero. Even in the early universe.
"Thermodynamics during that age determined the lowest achievable possible temperature, not you."
Yup. Good job I don't.
"I call that thermodynamically lowest possible temperature "absolute zero"."
You don't determine absolute zero.
Absolute zero is, and was 0K.
"If the universe expanded this "absolute zero" changes with it."
No, it doesn't.
The temperature of the universe reduces as it expands faster and faster. This is NOT absolute zero.
"Do you really think this epoch where we live in is so special, that we can only now come so close to "absolute zero"?"
It's been 15 billion years expanding quicker and quicker. Nothing special needed.
Absolute zero is 0K. It was 0K and it still is 0K.
"I already answered your earlier question about warm and cool being relative. "
You didn't answer my question about whether the cooler cavity is warming the hotter body.
If you have a body that is at a stable temperature with 99.9W of energy pumped into it in a chamber at 1/10th the temperature of the hot body. If you place that body inside a large chamber that is 1/5th the temperature of the hot body, that body is now losing 100W of energy in radiation from the body, but the chamber is radiating 0.8W.
This means that the hotter body, despite being in a cavity that is cooler than itself, is losing a net energy of 99.2W whilst 99.9W is being pumped in. A net increase in energy input into the system of 0.7W.
Does this hot body heat up in the cool cavity?
I am NOT asking "which is hotter, the cool cavity or the heated body within", I'm asking "Does the body within the cavity get warmed because the cavity, though cooler than it, warms it?".
I've already said which is warmer. So why would I be asking which is warmer?
Answer the question I asked: Does this hot body heat up in the cool cavity?
@ WoW
"It's got nothing to do with what I want. It is absolute zero. EVEN IN THE EARLY UNIVERSE."
-How is that thermodynamically possible in the early universe?
You can only determine that with a reference to the future. In your case the observer from today (for instance yourself).
Why do you think it is not possible to have a lower temperature than â273.15 °C if the universe is still expanding in the future?
You determined the lowest possible "absolute temperature" thermodynamically achievable temperature with instruments during THIS epoch. You cannot be certain that there is no lower temperature possible in the future.
There is no "freeze" of movement of molecules or a "freeze" of time. This is only relative.
Your hotter or colder question was already answered in thread 32 by me. It depends on the observer (viewer = the point of reference).
what to call hot or cold? There is no absolute cold or hot. It needs a (time) frame of reference.
What you call redshift I call Time Dependency.
Your question "Does this hot body heat up in the cool cavity?" has only a logical meaning if there is an absolute frame of reference. You do not state the observer(s) position measuring.
@WoW
Maybe you think I am completely against you.
No, I a not. Within your line of view you are completely correct. And I have not seen any more capable defender of your view and thoughts. And I totally agree!
The only problem with an absolute minimum temperature is that if there would be an absolute time independent temperature the expansion of this universe would come to an absolute stop.
That is because time will come to a halt too (no additional decrease in movement possible). It cannot cool anymore so the creation of space will stop abruptly. You can however introduce some kind of energy representing the void. This would slowdown the process.
"-How is that thermodynamically possible in the early universe?"
The same way it is possible in this one to get below the temperature of the universe at 2.7K.
"You can only determine that with a reference to the future."
Nope. Since the radiation laws depend on ABSOLUTE temperature, if the minimum temperature (Absolute Zero) were different, then the early universe would not have worked.
"Why do you think it is not possible to have a lower temperature than â273.15 °C if the universe is still expanding in the future?"
Because it isn't possible. At 0K there is 0W radiated from a body and unless it radiates, it can't lose any more energy.
How do you "know" absolute zero is reducing? Idiot.
"You cannot be certain that there is no lower temperature possible in the future."
Yes we can, far more certain than you are that absolute zero will get lower. If that does happen, then the stars will radiate quicker and in the past radiated slower. This would require a change in the evolution of the universe that would be seen.
"Your hotter or colder question was already answered in thread 32 by me."
No you haven't. This:
"It depends on the observer (viewer = the point of reference)."
for example, is complete bollocks and doesn't answer the question anyway.
Does the body with 99.9W going in and 99.8W coming out warm up?
It's got NOTHING to do with the observer.
""Does this hot body heat up in the cool cavity?" has only a logical meaning if there is an absolute frame of reference."
Listen you retard, that question IS about relative temperatures!
DOES IT GET HOTTER IS A RELATIVE QUESTION.
You just can't answer it because you're wedded to the idea that insulation violates the laws of thermodynamics, and your squirming around to avoid answering shows that you actually KNOW that you're talking bullshit.
Does that body warm up if there's more energy going in than comes out?
Yes or no.
"Maybe you think I am completely against you."
No, I think you're completely nuts. Absolutely, monumentally, stupid.
"The only problem with an absolute minimum temperature is that if there would be an absolute time independent temperature the expansion of this universe would come to an absolute stop. "
Care to prove how?
You see, that statement there is one piece of evidence that you're completely braindead.
If absolute temperature exists, then this doesn't stop the universe. Gravity would do that, not temperature.
If absolute temperature decreased, the radiation for the same body at the same temperature would increase and then fusion would eventually be unable to keep the temperature up and that WOULD stop any activity in the universe: there would be an accelerating heat death of the universe.
However, go ahead and show the figuring about how absolute zero MUST be changing with time. Include both the reasons that this not happening would cause the universe to stop and what evidence for this happening you have.
@WoW
"Absolutely, monumentally, stupid."
You confirm again your brain is limited to absoluteness only.
I am sorry to inform you that a further discussion with you is useless.
No, I asserted with evidence that you're a nutcase.
That you haven't managed to show any reason why your whacko lunatic ideas about "absolute zero" being time-dependent, any reasoning why the universe would stop if that were not the case, nor any even vague reasons behind any of your other ridiculous assertions, AND your failure to answer a simple question indicate part of the proof of my statement.
YOU discussing your lunacy with anyone other than a fellow religious nut or a psychiatrist IS useless. Not because of the other people, but because your ideas are ridiculous and you are unable to support them.
In other words, the problem is YOU. Not everyone else.