"The sun is a mass of incandescent gas
A gigantic nuclear furnace
Where hydrogen is built into helium
At a temperature of millions of degrees" -They Might Be Giants
It's so ingrained in us that the Sun is a nuclear furnace powered by hydrogen atoms fusing into heavier elements that it's difficult to remember that, just 100 years ago, we didn't even know what the Sun was made out of!
The conventional wisdom at the time, believe it or not, was that the Sun was made out of pretty much the same elements that the Earth is! Although that might seem a bit absurd to you, consider the following piece of evidence.
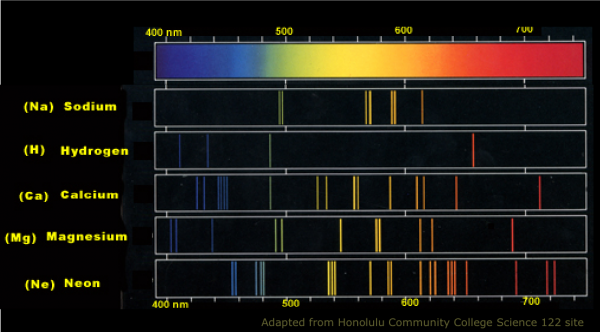
Every element in the periodic table -- which was well-understood back then -- has a characteristic spectrum to it. When those atoms are heated up, the transitions back down to lower-energy states cause emission lines, and when a background, multi-spectral light is shone on them, they absorb energy at those very same wavelengths. So if we observed the Sun at all these individual wavelengths, we could figure out what elements were present in its outermost layers by its absorption features.
That technique is known as spectroscopy, where the light from an object is broken up into its individual wavelengths for further study. When we do this to the Sun, here's what we find.
Basically, there are the same elements that we find on Earth. But what is it, exactly, that causes those lines to appear with the relative strengths that they appear. For example, you may notice that some of these absorption lines are very narrow, while some of them are very, very deep and strong. Take a closer look at the strongest absorption line in the visible spectrum, which occurs at a wavelength of 6563 Ångströms.
What determines the strength of these lines, as well as the relative weakness of the lines surrounding it? It turns out that there are two factors, one of which is obvious: the more of an element you have, the stronger the absorption line is going to be. That particular wavelength -- 6563 Å -- corresponds to a well-known Hydrogen line.
But there is a second factor that must be understood in order to get the strength of these lines right: the level of ionization of the atoms present.
Different atoms lose an electron (or multiple electrons) at different energies. So not only do different elements each have a characteristic spectrum associated with them, they can exist in a number of different ionized states (missing one electron, or two, or three, etc.) that each have their own, unique spectrum!
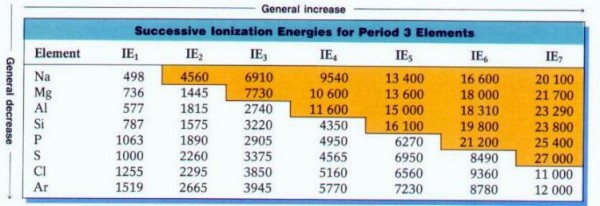
Image credit: Avon Chemistry, from http://www.avon-chemistry.com/, energies in kiloJoules.
Because energy is the only thing that determines the ionization state(s) of atoms, this means that different temperatures will result in different relative levels of ionization, and therefore, different relative levels of absorption!
So when we're looking at stars -- like the Sun -- we know that they come in a huge variety of different types, as a look through any telescope or binoculars will immediately show you, if it isn't clear to your naked eye.
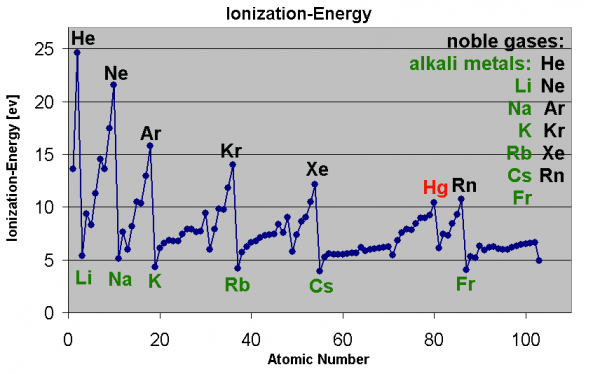
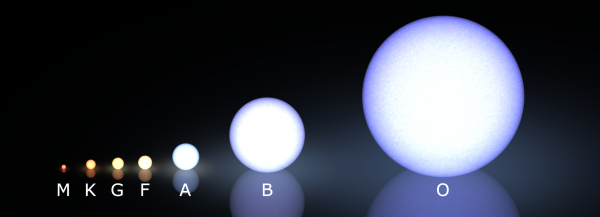
These stars, very notably, come in strikingly different colors, which tells us that -- at least at their surfaces -- they exist at vastly different temperatures from one another! Because hot objects all emit the same type of (blackbody) radiation, when we see stars of different colors, we're really detecting a temperature difference between them: blue stars are hotter and red stars are cooler.
After all, this is why we classify stars the way we do in modern times, with the hottest, bluest stars (O-type stars) at one end and the coolest, reddest stars (M-type stars) at the other.
But this was not how we always classified stars. There's a hint in the naming scheme, because if you had always classified stars by temperature, you might expect the order to go something like "ABCDEFG" instead of "OBAFGKM," right?
Well, there's a story here. Back before this modern classification scheme, we instead looked at the relative strengths of absorption lines in a star, and classified them by what spectral lines did or did not show up. And the pattern is far from obvious.
Different lines appear and disappear at certain temperatures, as completely ionized atoms have no absorption lines! So when you measure an absorption line in a star, you need to understand what its temperature is (and hence its ionization properties are) in order to rightfully conclude what the relative abundances of the elements are within it.
And if we go back to the Sun's spectrum, with the knowledge of what the different atoms are, their atomic spectra, and their ionization energies/properties, what do we learn from that?
That, in fact, the elements that are found on the Sun are pretty much the same as the elements found on Earth, with two major exceptions: Helium and Hydrogen were both vastly more abundant than they are on Earth. Helium was many thousands of times richer on the Sun than it is here on Earth, and Hydrogen was about one million times more abundant on the Sun, making it the most common element there by far.
Know who the scientist was who put this all together? I'll give you a hint: it was a 25-year-old woman who was never fully given the credit she deserved.
Meet Cecilia Payne (later Cecilia Payne-Gaposchkin), who did this work for her Ph.D. thesis way back in 1925! (Astronomer Otto Struve called it "undoubtedly the most brilliant Ph.D. thesis ever written in astronomy.") Just the second woman to earn her Ph.D. in astronomy through Harvard College Observatory (where she had to move to earn one; her original alma mater, Cambridge, didn't award Ph.D.s to women until 1948), she wound up having a remarkable astronomy career, becoming the first female chair of a department at Harvard and an inspiration to generations of astronomers, both male and female.
Historically, Henry Norris Russell (of Hertzsprung-Russell fame) was often given the credit for the discovery that the Sun is primarily composed of hydrogen, as he dissuaded Payne from publishing her conclusion and stated it himself four years later. Let that be the case no longer! This was Cecilia Payne's brilliant discovery and she deserves full credit for making it. The strength of the absorption lines combined with the temperature of the stars and the known ionization properties of atoms leave you with the inescapable conclusion: the Sun is a mass of primarily Hydrogen! Thanks to Cecilia Payne, now you know how we know that.








I have a slightly pedantic trivia fact check for you: "Why Does the Sun Shine" was recorded by TMBG, but it was written by Hy Zaret way back in 1959. It is not so great an achievement as Cecilia Payne's, but... credit where credit's due and all that.
Also, TMBG has since recorded an update to their song, entitled "Why Does the Sun Really Shine", where they make a correction: "The sun is miasma of incandescent plasma", and also comment that the previous "thesis has been rendered invalid". http://www.youtube.com/watch?v=sLkGSV9WDMA
One of TMBG's science songs is a *cover*?! Amazing!
Not as amazing as Cecilia Payne's discovery!
Sad how men fought so hard to prevent women from achieving in science, and when they went and did it anyway, fought to keep their names out of the history books. Very similar thing happened to Rosalind Franklin wrt the discovery of DNA's structure. She's the one who actually did the work to actually figure it out. But then some schmucks took all the credit and tried to relegate her to the historical dust bin.
So far so good on digging them out, and thanks Ethan for contributing! I only wonder at all the women -- and, possibly, the discoveries by them -- that are still unknown.
Also, when TMBG learned about the factual errors in "Why does the Sun Shine", they wrote their own song called "Why does the Sun Really Shine":
http://www.youtube.com/watch?v=sLkGSV9WDMA
Still doesn't explain "OBAFGKM" ;)
Soylent green.
Which is made from humans.
Which are made of ex-star stuff.
It's a cycle of nature!
(and there's no explanation for why astronomers have a mnemonic "Oh Be A Fine Girl, Kiss Me, Right Now! Smack!" probably made up by an Arts Major as ironic humour...)
Fun fact: the corona has a density 12 orders of magnitude less than the earth's atmosphere.
So, if you wanted to describe what that first picture of Ethan's is made of, a reasonably accurate answer is: nothing. That big orange surface, all those beautiful filaments? Mostly empty space.
Sure, but why is our standard of not-nothing earth atmosphere? At the sub-atomic level the iron core of the earth is still mostly empty space. :)
In your last paragraph, as you have phrased it, it seems (or at least
I understood it that way when I read it) as if Russell just parroted what
Payne said and took the credit for it. What happened is that later Russell
arrived at the same conclusion using a different argument, which led to its
acceptance; and he recognized Payne's earlier work in his 1929 paper.
CB: Also Lisa Meitner did most of the work which led to the discovery of fission of uranium. The person who got all the credit, and a Nobel Prize, was her colleague Otto Hahn.
And many people think Einstein's wife (name escapes me), who he himself admitted did the checking of his mathematics, did a lot of the work leading to his seminal papers on relativity, the photoelectric effect, and brownian motion (all published 1905).
"Sad how men fought so hard to prevent women from achieving in science"
"fought"
I like the assumption that this is no longer the case.
However, your statement implies there's been no noticeable improvement.
This isn't the case either.
Is the colour of the inside of the sun different to the outside of it due to the temperature difference?
Is the inside blue and the exterior red?
John Armstrong
It seems that Pickering's assistant Fleming started a star classification that went through the alphabet from A to V. Stars with the most hydrogen were A; then B, then so on up to V. But it was a clumsy scheme.
So, " In 1901, another of Pickering's assistants, Annie Jump Cannon, also begun to work on the classification sequence. Her meticulous observations led her to simplify the 22-type scheme into a simple sequence of *temperature*: OBAFGKM."
The OBAFGKM stars were the most important stars because they organized the stars by surface temperature. The other star letters seem to have either disappeared (absorbed as subsets of the ( OBAFGKM) or originally served specialized stars. e.g. L and T are brown dwarfs, P for Planetary nebula, Q for nova. I see Y as another type of Brown drawf (so maybe the alphabet scheme was used for more than 22 letters).
I'm not a historian, so I defer to any who knows this history better. So Annie Jump Cannon was another woman astronomeer. "She is credited with the creation of the Harvard Classification Scheme (OBAFGKM), which was the first serious attempt to organize and classify stars based on their temperatures... The female astronomers doing this groundbreaking work at Harvard Observatory earned 25 cents per hour, which was less than what the secretaries at the university earned." wikipedia
Cannon's work came before Cecilia Payne's work.
http://www.sdsc.edu/ScienceWomen/cannon.html
What is the Sun made out of? Iron, if you're a crank, or easily persuaded by one.
When we talk about those denied credit for their work, let's not forget Jocelyn Bell Burnell, who should have at least shared the 1974 Nobel Prize for her discovery of the first radio pulsars. She did get a belated knighthood, and inspired thousands of young women scientists.
this does not answer my question..... WHAT IS SUN'S ENERGY MADE UP OF ????
Because your question makes no sense, madoca.
Anyone trying to answer will have to make sense of it because as written it's nonsense. And therefore they will answer a question they have tried to work out as being the one that you'd say if you knew how to say it.
You'd be as well off demanding "WHAT IS FOOD'S ENERGY MADE UP OF????" then complaining when it said to be "chemical energy" that "this does not answer my question" because you didn't mean that.
i thought the sun was made from plasma
And your house is made out of solid, your drink is made of liquid?