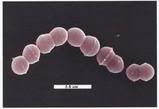
 I wrote here that pili--long, filamentous surface molecules involved in adhesion and bacterial "sex"--had recently been discovered in gram positive organisms; pecifically, in group A and B streptococci (Streptococcus pyogenes and Streptococcus agalactiae, respectively), using a genomics approach. Though this publication is quite recent, this is a fast-moving area of research, as evidenced by two new papers which extend this earlier research into pili in the group B streptococcus (GBS).
I wrote here that pili--long, filamentous surface molecules involved in adhesion and bacterial "sex"--had recently been discovered in gram positive organisms; pecifically, in group A and B streptococci (Streptococcus pyogenes and Streptococcus agalactiae, respectively), using a genomics approach. Though this publication is quite recent, this is a fast-moving area of research, as evidenced by two new papers which extend this earlier research into pili in the group B streptococcus (GBS).
A new study published in Molecular Microbiology further analyzes the role of pili in GBS. This is a bacterium that was originally identified as a cause of bovine mastitis (infection of the mammary glands), and subsequently found to be a significant cause of neonatal meningitis and other invasive infections. Though much attention has been paid to the general epidemiology of this organism, with many studies carried out examining risk factors for colonization and invasive disease, serotype distribution, and antibiotic resistance, less is known about specific virulence factors that contribute to disease, and there is no vaccine available. This paper focused mainly on the assembly of the pili, which I won't delve into, but they also examined the role of the specific pili genes in adhesion to human epithelial cells. They found that adherence wasn't affected when genes were deleted encoding for the major pilin or genes that affected polymerization of the pilin subunits, but mutants with a deletion of a single pilin-associated gene, Gbs 1478, were significantly reduced in their ability to adhere to human cells. Gbs 1478 is a gene encoding a protein which contains an LPXTG motif, which is characteristic of surface proteins in gram-positive bacteria. Therefore, they suggest that 1) this is the pilin-associated adhesion, and 2) the activity of the adhesion isn't dependent upon pilin synthesis.
So, this suggests that the genes that encode the pilin may play a role in infection--specifically, in adherence to epithelial cells, which is generally a requirement for infection. This brings me to the second paper.
As I mentioned above, there's no vaccine against S. agalactiae. One reason for this is because the most immunogenic portion of the bacterium is its polysaccharide capsule, which envelopes the bacterium and contains the antigens that result in generation of serotype-specific antibodies. The problem is that polysaccharides don't often work well as vaccine candidates; they are poorly immunogenic in infants and children, who are often the population targeted for such vaccines. To get around this, polysaccharides can be coupled to another molecule (generally an immunogenic protein); this increases the immune response and therefore, the effectiveness of the vaccine. This strategy has been used in the vaccine against Haemophilus influenzae type b (Hib), for example. It's trickier for streptococci, however, because there isn't a single capsule type that causes all disease. A related vaccine against Streptococcus pneumoniae contains antigens for 23 different capsule types (a "23-valent" vaccine). GBS has only 9 major capsule types, and only 5 of them are commonly found in the U.S., but a vaccine that uses a single antigen present in all forms of the bacteria is preferable. This is where the pili come in.
Pili have previously been demonstrated to be antigenic, and have been found to confer protection when used as an intranasal vaccine in mice who were later challenged with Streptococcus pyogenes.. In a new JID paper, a different strategy is utilized, inserting the pili genes from the group B streptococcus into a non-pathogenic strain of bacterium commonly used as a probiotic, Lactococcus lactis, and using this recombinant strain as a live vaccine in mice.
The authors were most concerned with using the vaccine to protect neonates (as in humans, antibodies against GBS are transferred across the placenta to the fetus, and provide some measure of protection during the early months of life). Therefore, they immunized female mice with a subcutaneous dose of the recombinant Lactococcus, and then examined survival of their offspring following challenge with a lethal dose of S. agalactiae. They found that more than 70% of the mouse pups survived, which was similar to results obtained when a protein-based vaccine alone was used. Pups were also protected when the mothers were inoculated intranasally rather than subcutaneously, although a significantly higher dose was necessary to achieve protection.
S. agalactiae currently is controlled by screening women during the late stages of pregnancy. Women who test positive for the bacterium (which is carried asymptomatically in the vagina or rectum in up to 35% of healthy women) are given antibiotics during labor in order to prevent the transfer of the bacterium from the woman to the neonate during birth. This has been an effective intervention, but it has problems, including the risk of increasing antibiotic selection pressures; colonization with a resistant strain; and risk of transfer of the organism prior to birth (via ruptured membranes, for example). Carriage can also be transient, meaning that although no S. agalactiae may be found upon routine screening, the woman may be infected at the time of birth. An effective vaccine could reduce a number of these risks, and more efficiently protect neonates from infection with this organism. Additionally, S. agalactiae also affects the elderly, diabetes patients, and others who are in some way immunocompromised, and a vaccine could also significantly reduce morbidity due to this bacterium in these risk groups as well. We're still a way off from using this as a human vaccine, but these studies certainly show the advantage of having sequenced genomes, and how "fishing expeditions" can quickly move into the realm of hypothesis-driven science with clinical applications.
References
Buccato et al. 2006. Use of Lactococcus lactis expressing pili from group B streptococcus as a broad-coverage vaccine against streptococcal disease. Journal of Infectious Diseases. 194:331-40. Link.
Dramsi et al. 2006. Assembly and role of pili in group B streptococci. Molecular Microbiology. 60:1401-13. Link.
Image from http://www.umb.no/cache/f7def7782f1050bc4765b86126d21355.jpg

Hi, dumb science question. You mention several times in these articles that you are referring to "gram-positive" bacteria. What is the significance of this term? Wikipedia explains only that "gram-positive" bacteria possess a cell wall layer which makes it possible to stain the bacterium blue. I am just curious, what is it that leads you to specifically point out that the bacteria in these articles are gram-positive? Is there something about gram-positive bacteria which makes them more interesting from a research perspective than other kinds of bacteria?
Hi Coin,
That's not a dumb question at all. When we look at bacteria, we separate them using a few basic criteria. One, their shape--are they round ("coccus") like strep? Are they rod-shaped, like E. coli? Are they twisty, like Borrelia (called "spirochetes")? Another is via the gram stain, which essentially divides bacteria into two camps: gram-positive and gram-negative, based on their cell wall structure as you mention. This is important because it serves as the basis for determining the species of bacteria ("gram positive coccus" like strep, "gram negative rod" like E. coli, etc.), and because some antibiotics target one class of bacteria or the other, so it's important to know a bit about the physiology of the organism.
Additionally, this is thought to be an ancient split between bacterial lineages, so generally, gram-negatives are more closely related to each other than they are to gram-positives, and vice versa. It's kind of like dividing humans up by race/ethnicity--it doesn't tell you everything you want to know, but it's a pretty quick and easy starting point.
Tara,
I still remember sitting in Mark Wheelis' introductory microbiology class at UC Davis and asking about why gram positive bacteria did not have pili. Mark was always so forward-thinking. He said that there were no reasons why gram positive bacteria shouldn't have them, but no one had observed them to date. "Some day someone is almost certainly going to find a gram positive prokaryote that has pili," he said.
Now we know, Mark was right. This is cool stuff, because pili are so important for epithelial surface colonization. Making clean vaccines against them is easy and effective.
Cool stuff!!
Tara,
does anyone know what residues these pili bind to? (this is from someone who used to study FimH in E. coli, so lay it on thick...)
Just a quick cautionary note on the analogy with race and ethnicity above. Dividing the bacteria into these categories reflects actual lineage-based biological differences apparently, ie the groupings reflect an objective category that has biological reality. Race and ethnicity on the contrary are socially/culturally constructed categories that do not reflect objective biological categories. Population genetics tells us that the so-called "races" of humans are actually relatively arbitrary groupings (from a genetic point of view) based on the sociopolitical selection of a handful of phenotypic characters such as skin color, not on actual genetic/biological similarity. If one arbitrarily chose genetic markers for lactose intolerance rather than melanin production (skin color)then "white" Scandanavians would be the same race as "black" south africans, while Italians and Greeks would be the same race as central to north black africans. This all is probably very familiar to you already, but for your readers sake I thought it was worth posting. I am constantly amazed at how many well-educated, even scientifically educated adults stilll tend to think of race as a biological fact rather than a social construct. Such is the legacy of slavery and racism.
sylvilagus,
I knew I was going to get in trouble with the race analogy. :) (That's why I put in race/ethnicity, to kind of ward that off...) Actually, due to horizontal transfer, we see some of what you describe in bacteria as well--depending on the genetic marker used, different groupings can be created, but the gram +/gram- is still the most useful as an initial rough characterization.
Mike,
That work may be in progress, but they don't mention what they bind to in the current paper. All they say is that they saw similar results using A549 cells (pulmonary epithelial cells) and HeLa (cervical) cells.
"I still remember sitting in Mark Wheelis' introductory microbiology class at UC Davis and asking about why gram positive bacteria did not have pili. Mark was always so forward-thinking."
Talking of Mark Wheelis, his recent book "Deadly Cultures" on the history of biological warfare, is the Dog's Bollocks.*
(*That's UK slang for 'extremely good')
Hi,
Do you think there could be a connection between group b strep acquired infection in utero and subsequent development of PANDAS ( paediatric auto immune neuro developmental disorders) such as tic disorder and obsessive compulsive disorder. At the moment it's thought this can be due to acquired infection with group a strep in childhood.
Regards
Anne Berrich