**A post about Climate Change as a part of Blog Action Day 2009**![]()
 When people talk about climate change, they, more often than not, talk about global warming. Yes, the effects of increased temperature will be diverse and generally bad for most creatures on Earth, including us. But the most dramatic effect of climate change won't be due to the heat - it will be due to ocean acidification. I might seem biased (being a marine biologist and all), but trust me, the addition of carbon dioxide to the ocean and its subsequent effects will be far worse in the long run than a change in temperature. Not so sure? Let me explain.
When people talk about climate change, they, more often than not, talk about global warming. Yes, the effects of increased temperature will be diverse and generally bad for most creatures on Earth, including us. But the most dramatic effect of climate change won't be due to the heat - it will be due to ocean acidification. I might seem biased (being a marine biologist and all), but trust me, the addition of carbon dioxide to the ocean and its subsequent effects will be far worse in the long run than a change in temperature. Not so sure? Let me explain.
 I know for most of you it's been a long time since you took a chemistry course, so here's a quick refresher. Acidity of a solution, or its "pH", is a measure of the concentration of hydrogen ions (H+). The lower the pH, the more acidic, and the higher the concentration of H+. The effects we associate with acidity - burning through flesh, for example - are due to the fact that H+ ions are extremely reactive with other molecules, and tend to incite chemical reactions.
I know for most of you it's been a long time since you took a chemistry course, so here's a quick refresher. Acidity of a solution, or its "pH", is a measure of the concentration of hydrogen ions (H+). The lower the pH, the more acidic, and the higher the concentration of H+. The effects we associate with acidity - burning through flesh, for example - are due to the fact that H+ ions are extremely reactive with other molecules, and tend to incite chemical reactions.
The astute reader might note here that CO2 doesn't contain H+ ions, or hydrogen at all, for that matter. How can it cause seawater to become more acidic?
The key is in how carbon dioxide reacts with water when it is dissolved. Unlike other gasses, CO2 doesn't stay in its gaseous form when it becomes aqueous, it reacts almost instantly with water to form H2CO3. In turn, this compound, called carbonic acid, tends to release its two hydrogen ions in sequence, becoming bicarbonate and carbonate:
H2O + CO2 -> H2CO3 -> HCO3- + H+ -> 2H+ + CO32-
It's easy to see how adding CO2 into the equation would drive the production of hydrogen ions. But, in reality, the reaction is more complex than that. Because seawater is a slush of ions, it's in what is called chemical equilibrium, where ions back and forth between the different forms. This gives seawater natural buffering, which means that adding an acid doesn't directly raise its pH. Extensive experiments have shown that adding CO2 doesn't just make more hydrogen ions and CO32-. How much CO2 remains as CO2, HCO3- , and CO32- is influenced by a number of other factors, including the water's temperature and alkalinity.
The equilibrium looks much more like this:
H2O + CO2 + CO32- HCO3-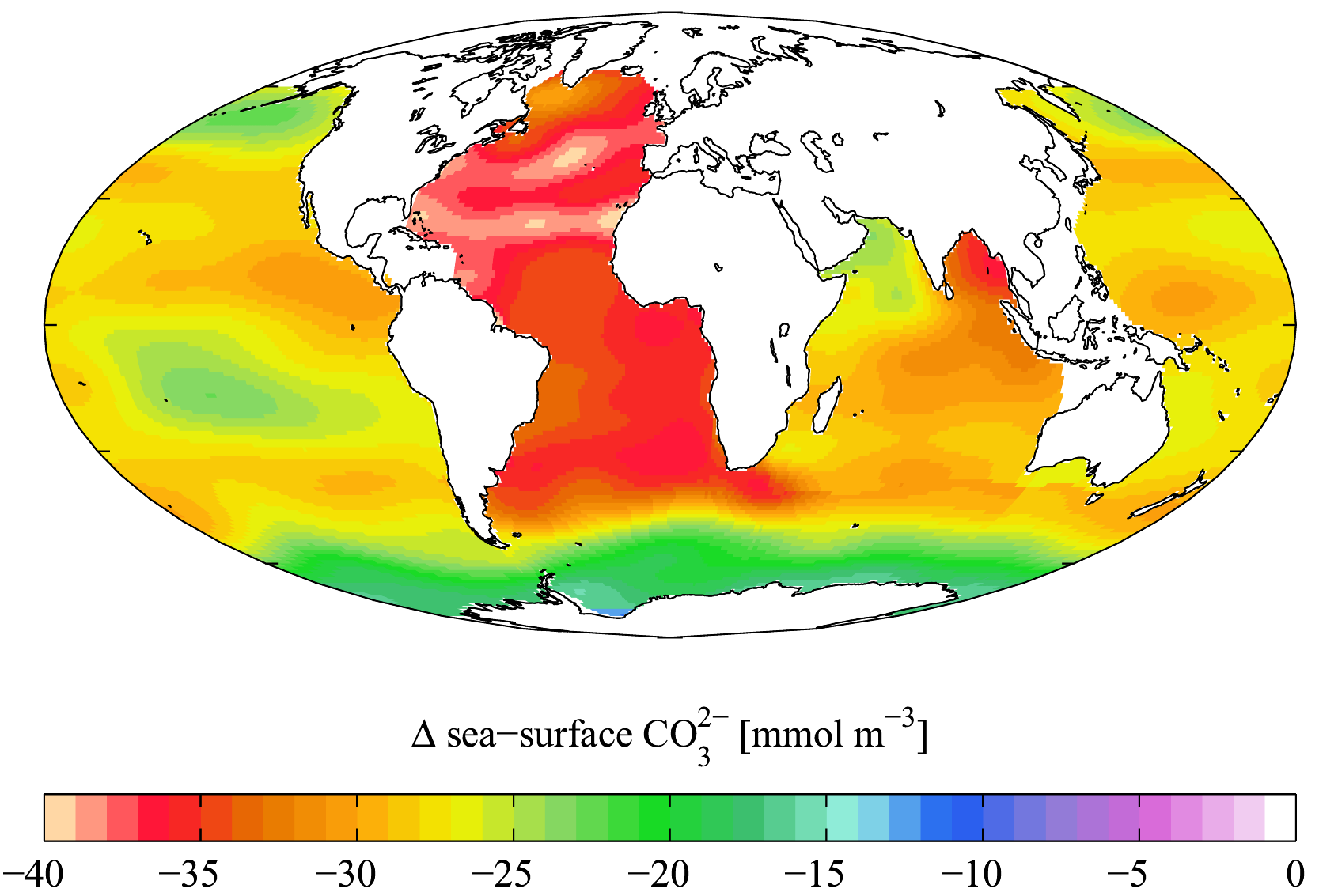 The majority of carbon dioxide in seawater ends up as HCO3-. As more CO2 is added, it reacts not only with water to produce HCO3- + H+, but those hydrogen ions, in turn, react with CO32- to create HCO3-. The figure to the right shown the overall change in concentration from 1750s to the mid 1990s; during that time, surface ocean pH is estimated to have decreased from approximately 8.179 to 8.104, which corresponds to about a 20% increase in the hydrogen ion concentration. If you're really interested in the complex chemistry, check out K. G. Schulz et al's paper that just came out in 2009 - it delves deeply into exactly how this works (and when I say deeply, I mean deeply - good luck if you're not a chemist).
The majority of carbon dioxide in seawater ends up as HCO3-. As more CO2 is added, it reacts not only with water to produce HCO3- + H+, but those hydrogen ions, in turn, react with CO32- to create HCO3-. The figure to the right shown the overall change in concentration from 1750s to the mid 1990s; during that time, surface ocean pH is estimated to have decreased from approximately 8.179 to 8.104, which corresponds to about a 20% increase in the hydrogen ion concentration. If you're really interested in the complex chemistry, check out K. G. Schulz et al's paper that just came out in 2009 - it delves deeply into exactly how this works (and when I say deeply, I mean deeply - good luck if you're not a chemist).
The problem is, many organisms, including corals and photosynthetic algae, need CO32- to form the hard shells that they live in. They use dissolved calcium (Ca2+) along with the dissolved carbonate (CO32-) to create calcium carbonate (CaCO3). They cannot use bicarbonate (HCO3-). So as the levels of carbonate drop, it becomes harder and harder for organisms to make calcium carbonate.
This is bad news for coral reefs. Coral reefs are the most biologically diverse ecosystem in the marine environment and are crucial for conservation, fisheries, tourism and coastal protection. They make up about 1/6th of the world's calcium carbonate production, currently producing 900 million tons a year. Scientists predict that the change in seawater chemistry could doom these fragile ecological hot spots, causing the loss of billions of dollars in the fishing industry alone.
But corals aren't the only species that could be affected by reduced carbonate. Small, unicellular amoeba called Foraminifera rely on carbonate. What they lack in size they make up for in impact; foraminifera, or "forams", are one of the most abundant organisms on earth, and create another 1.4 billion tons of calcium carbonate a year, around 25% of the grand total. Similarly, other planktonic creatures are dependent on carbonate, too, like pteropods. Pteropods are small molluscs that are found, in particular, in the southern oceans, where they are a food source for species like krill. As the base of the marine food web in many areas, pteropods are key to the overall health of our oceans, and have been called the "canaries in the coal mine" for ocean health.
Pteropods are small molluscs that are found, in particular, in the southern oceans, where they are a food source for species like krill. As the base of the marine food web in many areas, pteropods are key to the overall health of our oceans, and have been called the "canaries in the coal mine" for ocean health.
It's not just theory that an increase in atmospheric carbon dioxide relates to a drop in carbonate levels that will hurt these species. Scientists have shown that as carbon dioxide levels rise, the acidity of the ocean is changing, and it is slowing, if not stopping, these organisms from growing. Multiple studies have shown that corals to be inversely affected by increased carbon dioxide and decreased carbonate ions, though the effects do vary. Acidification also harms all kinds of carbonate plankton, larger carbonate organisms like sea urchins, and even certain species of algae. Noise travels further and faster as seawater pH drops, which may impact all kinds of organisms, including fish. But the most devastating loss will likely be in planktonic primary producers like pteropods. A study published in Nature showed that not only do pteropods suffer in reduced carbonate availability, computer modeling suggests that the majority of the southern ocean will become pteropod-uninhabitable by 2100 if we keep emitting carbon dioxide at the rate we do now.
While rising temperatures and melting ice are nothing to sneeze at, completely decimating the base of the marine food web is going to be disastrous. Carbonate-requiring organisms are primary producers which are responsible for the majority of the ocean's estimated 90% contribution to the world's oxygen production - which means the loss of them could have a more devastating impact on atmospheric CO2 than chopping down all of the world's forests. And that's not even taking into account the loss of biodiversity that will ensue as we take away the bottom of the food chain. The economic losses from decreased fish catch and tourism will be easily in the billions a year.
Climate change is a major issue that our generation and those that follow need to address - but let's remember that it's not just about the heat. Acidification and other affects of carbon output are likely to make an even bigger impact. We need to asses, monitor, and propose solutions for all of the effects of climate change. Now is the time to protect our future - not only on land, but in the water, too.
References
K. G. Schulz, J. Barcelos e Ramos, R. E. Zeebe, & U. Riebesell (2009). CO2 perturbation experiments: similarities and differences between dissolved inorganic carbon and total alkalinity manipulations Biogeosciences, 6, 2145-2153
LANGER, M. (2008). Assessing the Contribution of Foraminiferan Protists to Global Ocean Carbonate Production The Journal of Eukaryotic Microbiology, 55 (3), 163-169 DOI: 10.1111/j.1550-7408.2008.00321.x
Marubini, F., Ferrier-Pagès, C., Furla, P., & Allemand, D. (2008). Coral calcification responds to seawater acidification: a working hypothesis towards a physiological mechanism Coral Reefs, 27 (3), 491-499 DOI: 10.1007/s00338-008-0375-6
Gattuso, J. (1998). Effect of calcium carbonate saturation of seawater on coral calcification Global and Planetary Change, 18 (1-2), 37-46 DOI: 10.1016/S0921-8181(98)00035-6
Riebesell, U., Zondervan, I., Rost, B., Tortell, P., Zeebe, R., & Morel, F. (2000). Reduced calcification of marine plankton in response to increased atmospheric CO2 Nature, 407 (6802), 364-367 DOI: 10.1038/35030078
Fine, M., & Tchernov, D. (2007). Scleractinian Coral Species Survive and Recover from Decalcification Science, 315 (5820), 1811-1811 DOI: 10.1126/science.1137094
Kuffner, I., Andersson, A., Jokiel, P., Rodgers, K., & Mackenzie, F. (2007). Decreased abundance of crustose coralline algae due to ocean acidification Nature Geoscience, 1 (2), 114-117 DOI: 10.1038/ngeo100
Orr, J., Fabry, V., Aumont, O., Bopp, L., Doney, S., Feely, R., Gnanadesikan, A., Gruber, N., Ishida, A., Joos, F., Key, R., Lindsay, K., Maier-Reimer, E., Matear, R., Monfray, P., Mouchet, A., Najjar, R., Plattner, G., Rodgers, K., Sabine, C., Sarmiento, J., Schlitzer, R., Slater, R., Totterdell, I., Weirig, M., Yamanaka, Y., & Yool, A. (2005). Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms Nature, 437 (7059), 681-686 DOI: 10.1038/nature04095
