Last night, I was watching the Daily Show, and they had Tom Zoellner on, talking about his new book: Uranium: War, Energy, and the Rock that shaped the world.
There are certainly a lot of interesting things about Uranium in culture, particularly in terms of energy (hey, we can use this thing to power the world) and in terms of war (the nuclear arms race). If you look at what we've done with all the enriched Uranium (U-235, the fissionable type), the US and the Soviets, from the 1940s to the 1980s, basically took nearly all of it and stockpiled it.
This is the only element on Earth we've ever done that to, and that makes it incredibly interesting from a cultural point of view.

But Tom Zoellner said something interesting during the interview about Uranium, that I'd like for you to think about with me:
[Uranium] is one of these things... it's the heaviest naturally occurring mineral in the periodic table. I mean, the table goes up higher, but this is where it ends. This stuff seems to hit some invisible wall of nature where it has this really big nucleus, this nucleus of 92 protons, and the center can't hold.
Now, this is mostly true. It is the heaviest naturally occurring element on Earth. We don't find any elements with 93 protons (Neptunium), 94 protons (Plutonium), or higher numbers except the ones we've made in laboratories. Here's a version of the periodic table of naturally occurring elements for you:

But -- here's a great question for you -- why? Why is Uranium the heaviest element on Earth? Well, there are two reasons: one is obvious, and one is very subtle. The obvious reason is that the elements shown above in light grey, 43-Tc, 61-Pm, and everything above and including 93-Np are unstable. They get created in stars and supernovae, just like all the other elements, but they radioactively decay into the other, stable elements (1-42, 44-60, and 62-92).
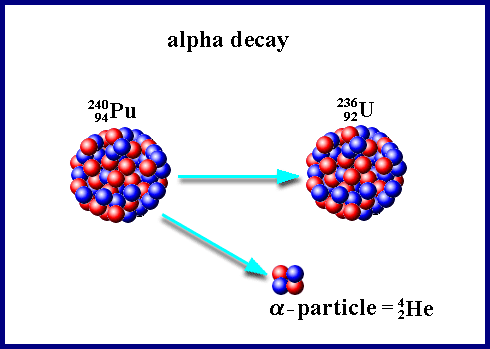
But hang on a second. Isn't Uranium, element 92, also unstable? Its most stable isotope, Uranium 238 (92 protons + 146 neutrons), has a half-life of about 4.5 billion years, or the age of the Earth. Well, that explains why there's still so much Uranium around: it's only had enough time for about 50% of the atoms to decay. The fissionable type of Uranium, Uranium 235 (3 fewer neutrons), is less stable, with a half-life of 700 million years, is still around, too. Much more of it -- about 98.8% -- is gone by now. However, there's still enough left that there's plenty on Earth.

But every element heavier than lead (element number 82) is unstable, and will eventually decay! Of all the unstable elements that aren't on Earth, though, I'm curious about why there isn't any Plutonium? Plutonium-244, with 94 protons and 150 neutrons, has a half-life of 83 million years, which means that there should still be small amounts of it left today. Plutonium is more stable than Protactinium and Actinium, which are found in Nature. Only one study, whose results are not universally accepted, claims to have detected natural Plutonium. So, now for the more subtle point: why isn't there any Plutonium-244 on Earth?

The only way you produce elements heavier than Iron-56 is in Supernovae: when massive, dying stars collapse and explode. These heavy elements then get spread out over a large area and recollapse, forming new stars and planets. That's where all of the elements on Earth came from. But the supernova that gave rise to us must not have been powerful enough to make huge amounts of Plutonium! Since there are supernovae that are up to 100 times more powerful than normal ones, there are explosions out there in the Universe that will litter it with Plutonium. We saw one in 2006 that probably did just that:

Well, the planets that get created from the dust of this explosion will not see Uranium (element 92) as their "wall of nature," they'll see Plutonium (element 94) as the heaviest, or possibly even Curium-247 (element 96)! There are people searching for this as well. And, on a lighter note, there will surely be planets out there where the heaviest element is lighter than Uranium, possibly even lighter than lead! So yes, Uranium is the heaviest natural element that we find on Earth, but there are certainly heavier ones out there!


Great explanation!
I'll say it's a great explanation. Now, how do I role this one out in casual conversation?
One thing I've been curious about is whether any of the elements in the Island of Stability theorized to exist far out on the Periodic Table past what we've ever seen before are ever created in Hypernova or some such. I don't know if it'd be possible, but it does make me curious as to whether anyone has ever looked before. I mean, if it was really delved into the Periodic Table should give some idea of what spectral emissions we'd see (It's the friggin' Periodic Table, afterall....), but I don't know if anyone ever has done this before.
great. just what we need. aliens visiting us from a planet with naturally occuring plutonium. they won't have tin-foil hats, they will have plutonium foil hats!
welcome to SB
The problem with the blogpost is that both neptunium and plutonium are found in very small quantities in the earth--that is to say, naturally-occurring isotopes of those elements are.
The best I can tell is that uranium is held to be the heaviest "naturally-occurring element" on earth because of tradition, convention, and the very small quantities of heavier isotopes being found. It's just traces of neptunium and plutonium that are found, after all, nothing anybody is going to mine. What is more, even heavier isotopes could be found "in nature," at least if one could get close enough to recent supernova II ejecta. What is more, the little bit of neptunium and plutonium found are presumably "built upon" uranium, or, perhaps in some cases, thorium.
The point is that the "heaviest naturally-occurring element" is an arbitrary designation, depending on where you cut off "nature." Naturally occurring neptunium and plutonium are simply traces that are being constantly produced at very low rates via nuclear interactions, and barely count at all.
Interestingly, I watched a program recently on National Geographic Channel which did count neptunium and plutonium as the heaviest "naturally occurring elements"--on earth, as I recall. I did not think it the best thing to do without explanation (there was none), since I do consider the term arbitrary, and the mere fact that a californium atom may occasionally settle upon the earth is about as unimportant as the tiny bits of naturally-produced neptunium and plutonium are found on earth.
That said, we shouldn't really make the following statement:
Glen D
http://tinyurl.com/6mb592
Thank you very much for that explanation. I watched the very same show and I was puzzled by that answer. I did not think it through but I am glad that you did. I teach high school physics and these are the types of interesting thought questions that get kids engaged.
Thanks for the post! Quite fascinating.
What I don't understand is this. I assumed that in a dying star, the heaviest element at the core will be Iron, and that anything heavier gets created in the supernova explosion. I assumed the energy of the supernova was enough to fuse nuclei together and create heavier elements. Correct me if I'm wrong...
So it seemed odd to me that the temperature of the supernova explosion happens to lie so finely between the fusion potentials of Uranium and Plutonium. I would have thought it likely that the explosive energy would overcome the fusion potentials for all kinds of nuclei, eventually leaving only stable elements in its wake. Obviously, your observation about Plutonium means my naive assumption was wrong!
How good are our supernova models? Can we really predict the heaviest nuclei we'll produce as a function of the star's mass? Or are we working in reverse and building supernova models from the Plutonium abundance?
Well I, for one, am quite pleased with what used to be a lack of plutonium on Planet zero, too bad we didn't leave it that way.
Now keep this low key, otherwise it will be yet another proof of the existence of god. I'm trying to get a couple of kids through school before science is outlawed in TX.
Yeah, I just looked at the periodic table, and Uranium is still around because it has a long-lived isotope. Plutonium doesn't. I'm skeptical of this idea. A larger supernova would make more stuff, but I don't see why the relative abundances would be any different.
Interesting, but don't forget that these actinides have had more than 4.5 billion years to decay, that started as soon as they were made and continued as the supernova remnants coalesced and became our favorite stellar system, time enough for a lot of unstable elements to decay into lead. And I never really thought of Hanford and Savannah River as labs, maybe really large production labs?
In case any heavy metal fans feel slighted, I will mention that this post is discussing atomic weight, not actual density. As any dedicated metal head knows, tungsten, gold, osmium, iridium and so on are all denser than uranium in evironmental conditions found at a typical heavy metal concert. This should always be borne in mind if you are carrying these elements in your pockets and wish to attempt stage diving.
Thanks for the great post. This will be a good topic of discussion with my kids.
Fissionable means capable of undergoing fission, which also applies to U-238. What you probably meant was fissile, which are the isotopes which will fission with the mere absorption of a neutron, without the requirement of any kinetic energy.
U-235 is fissile, while U-238 is not. They are both fissionable.
"This is the only element on Earth we've ever done that to,"
You appear to be neglecting gold! That's stockpiled up the wazoo!
I didn't know they had found and confirmed Plutonium on Earth as being natural. I was aware that they hadn't found it naturally, whereas they had expected it in significant trace amounts.
Why is there so little Plutonium? Well, 83 million years for a half-life is long, but 4.5 billion years is a long time. We would expect Pu-244 to be around in small but easily measurable abundances. But if it isn't there, we must have had trouble forming it. (If you want the details, you can look up r-processes of heavy element formation in supernovae, which I am not going to explain here.) But there are plenty of large and small explosions in space, and it's pretty reasonable that there are stars out there that produce larger abundances of the heaviest elements as well as ones that make smaller abundances.
Yes, when the Earth was only 1 billion years old, there was probably more Pu-244 than there is now, but there was also less than there would have been if it was produced in the same quantities as Uranium.
Yes, plenty of other elements are stockpiled, but there is still plenty of gold in the Earth. Uranium? Not so much...
As for the "island of stability"... show me some evidence for that one; until then, I'll say it's an interesting idea with no support.
From what I've read (not extensive), it's only Pu-239 that has been confirmed as occurring naturally. Below is not what I'd call an unimpeachable source, but one sees how a mix of uranium and beryllium could produce Pu-239, as explained there:
He seems not to realize that natural uranium doesn't produce many alpha particles to begin with, and then only around 30 neutrons per million alpha particles are produced by the beryllium. Nevertheless, a few neutrons would be produced, and uranium would capture those at reasonably good rates.
Another source I've encountered stated that, in their sample, spontaneous fission didn't seem sufficient to produce the neutrons needed to make the Pu-239 found, and, apparently, the sample was lacking in beryllium, or they hadn't thought about it. They contended that other sources had to account for their minute quantities of Pu-239, perhaps radioactive gases in the atmosphere, possibly cosmic rays.
That's interesting information about Pu-244. I hadn't heard that it should have persisted in measurable quantities had it been produced at expected rates. Sounds like one for the theoretical physicist, all right.
Glen D
http://tinyurl.com/6mb592
Well, I don't know if my last post is going to appear, but I went out and found one of the sources I mentioned there. I was wrong, it does discuss alpha particles producing neutrons in light elements (not necessarily beryllium) as a source of neutrons causing Pu-239 (and neptunium as well) to form, as well as spontaneous fission supplying the needed neutrons. Here's the abstract:
Glen D
Btw, most U-235 mined and enriched has been split in reactors. Stockpiles of both Russian plutonium and highly enriched U-235 have been fed into US reactors.
The highly enriched uranium was diluted down with unenriched (or depleted?) uranium for commercial reactors. Some Russian highly-enriched uranium has been saved for US subs and for possible future space reactors.
And by far the most U-235 is still in the ground. Nevertheless, all isotopes of uranium are about 1/10 as common in the entire earth as gold is. The reason it's relatively common for our purposes is that it is not siderophilic (so not in the core), nor is it a "compatible element" in the mantle. It's been estimated that 30 to 60 percent of the entire earth's uranium is in the earth's crust.
Glen D
http://tinyurl.com/6mb592
i need a element which is power full than uranim pls give in this website
I don't understand any of this.
please see about it at:
http://nanochemical.blogspot.com
thanks
83 million years is not very long for plutonium. That means ~99.999999999% of it is gone. Finding any would be a mighty chore.
There is still plenty of uranium around, which means uranium still decays. When uranium decays, what you have left is an element with fewer protons. Technically new atoms are created that are heavier than lead, but they only form from radioactive decay. That explains why elements that decay faster then plutonium are still around in vast quantities.
As an earlier commenter mentioned, there are still random atoms lying around which are heavier than uranium. Heck, there might still be an atom somewhere with 200 protons in it that hasn't decayed yet since decay is somewhat random. Finding one atom is nearly impossible though.
Also, immediately after a supernova, with that intense amount of energy, who knows how large of an atom is produced, there could have been 1000's of protons and electrons in some kind of equilibrium or semi-stable state. Perhaps it only existed for .00000000000000000000001 seconds, but it still existed.
uranium projects
naturally occurring plutonium is created near the lower layers of the earth where fission occurs with the uranium that's still buried deep. It is only small amounts though but that's still naturally occurring plutonium..it's being created under natural fissure.
Heavier elements? Well, according to some astronomical models, neutron stars are considered a single atom - neutrons and protons all squeezed together into a single lump, electrons combined with protons to form more neutrons or squirted out to who knows where. Same for black holes, although the math of what actually goes on inside breaks down. In that picture, there are not islands of stability, where atoms can have a slightly greater weight than the transuranium elements we have managed to synthesize here. Rather, it might be more fitting to speak of an island of instability, between iron as the heaviest stable small atom and the smallest possible neutron star as the lightest large atom, with an area of unstable elements between the two. And of course, there could be islands of stability inside this large region of instability. Pretty extreme cases though, where gravitational attraction is enough to overcome internal nuclear forces, and (for now, anyway) not commercially useful, where a single atom masses more than our entire solar system.
No telling at the moment, our physics is very much in its infancy with regard to such extreme conditions. Plenty of room yet for people with good ideas and a PhD. or Nobel prize in their sights.
we are talking about uranium 235 and i was wandering how long does it take for you to get more uranium 235?
Hey, I just came across this, and I've got to say, something here is really, really bothering me. Why did you use a periodic table that hasn't been accurate since 1993? I mean, even in 2009, digging up a modern and more accurate table would have been easy. That, combined with the fact that we do know for a fact that there have been and probably still are small naturally occurring deposits of plutonium made me quite skeptical that you actually know and understand this subject.
There actually are atoms of Plutonium-244 on earth, but they are exceedingly rare and are in very minute amounts.
you bunch nerds GOD created all u see and dont see in 6 literal days. stars dont create anything, believe the bible not scientific non-sense that cant ever be witnessed or proven they are mostly theory. Yes study the handy works he has created for man and creatures and give credit where its due.
@Anne #27: Ethan presented that table explicitly to show the _naturally_occurring_ elements. That set has not changed in a rather long time. All of the elements added to the table in the past 60 years are _not_ naturally occurring.
Why is it interesting form a cultural point of view? The reasons it was stockpiled by the military was obvious. The reason it was used for nuclear energy is even more obvious. It's not clear to me what is so interesting. It is a substance that has very useful properties in the ways mentioned. It being an element is completely incidental to its importance.
Ethan @15 and others: yes, we have discovered primordial plutonium on earth. See reference and bio below. Remember that it is not just a matter of finding gamma decays; in order to get a clean signal above the noise of other radioactive elements you need to separate the Pu from other rad elements enough to eliminate their background radiation. And when the plutonium is in the ppb range and expected to exist in thorium and uranium ores, that is extremely difficult to do chemically. Seeing it is not a physics problem, it's a chemistry problem. Solved by a chemist. :)
http://www.nature.com/nature/journal/v234/n5325/abs/234132a0.html
http://www.witi.com/center/witimuseum/halloffame/143609/Dr.-Darleane-C…
It is my great ambation to study nuclear camistry but unfortunatly it is not possible here!
The dissertation you provide is good as far as it goes, however it leaves a quite few things unexplained. For instance, why is Iridium denser than Uranium 238 if Uranium has a heavier nucleus than Iridium? Why are Manganese (At. # 25) and Tin (At. # 50) roughly the same density when Tin has a much higher atomic weight?
@John Charles Murray,
The simple answer is that density depends on two factors, namely mass and volume. You have considered only the mass part of the equation.
As for volume, while most of the atom's mass resides in the nucleus, most of the atom's volume comes from its electrons. This means that atomic radii are highly dependent on electron configuration. Consider sodium and potassium, for instance. The atomic weights of sodium and potassium are 23 and 39 respectively (rounding to whole numbers). By your logic, you would expect potassium to be much more dense than sodium. However, potassium has a single electron in its 4s orbital (in its ground state). The valence electron in sodium is in a 3s orbital. This makes the atomic radius of potassium much larger than that for sodium. Thus, the density of potassium is 0.862 g/cc while that of sodium is 0.971 g/cc.
Another factor also comes into play (and often dominates the atomic radius). We do not, and cannot, measure the density of a single atom of a given element. We measure the density of the element in its most stable bulk phase. For solids, this is a crystalline structure. Different crystalline structures result in different packing densities of the atoms. For instance, tin's crystalline structure is much more similar to carbon or silicon's tetrahedral packing structure, which is a more loosely packed structure, than the cubic or hexagonal close packing structures of most metals. Therefore, we would expect tin to have a lower density than would be indicated by its atomic weight alone.
No one seems to have this right. The heaviest naturally occurring element is element 98, Californium. 93-98 all get generated in tiny amounts continually from uranium. 2 of the elements below 92 only exist in very tiny amounts also. There is nothing magical about 92. A lot of old textbooks have this wrong. Wikipedia has a very good and accurate write-up on this.
Bobby - I've read the Wiki article but I'm not convinced. It talks about naturally occurring neutron capture and beta emission in Uranium creating Californium - but doesn't explain how this process can increase the atomic number and hence a heavier element. To my understanding it can only create a heavier isotope of Uranium that will decay to either a more stable isotope or a lighter element - not a heavier one
@Fraser #37: The combination of neutron capture followed by beta decay does, specifically, increase the atomic number by 1. Start with a nucleus (A,Z), where A is the atomic mass (total number of nucleons) and Z is the atomic number. Then neutron capture gives you (A+1,Z). Beta decay converts a neutron to a proton, so you get (A+1,Z+1).
If you are in a highly neutron-rich environment (like a nuclear reactor), then it is possible to have a series of neutron captures occur faster than the beta-decay half-lives, and you can walk up the ladder from (A,Z) to (A+n,Z+n), for n>1.
Fraser,
I think your confusion may be the result of your incorrect assumption that increasing the atomic number necessarily results in a heavier atom. A beta decay, for instance results in the emission of an electron, while at the same time increasing the atomic number of the atom that undergoes beta decay. The net result of this decay is conversion of a neutron to a proton. A proton has a slightly lower mass than a neutron, so the decay product would be lighter than the parent nuclide.
Bobby:
I'm skeptical. That's a six-neutron capture, which will be extroadinarily unlikely in just plain ore. Sure, I'll accept that some of those reactions probably occurred at Oklo a (literal) billion years ago, and that counts as 'natural,' but with a maxium half-life of 900 years for Cf-251, all of that is long gone.
I looked up the Wikipedia entry for Cf, per your suggestion Bobby, but the one reference it has to natural Cf is a book, not a peer reviewed journal article. Without more to go on, as I said, I'm skeptical. If we're seeing Cf in soil, I'd place my money on it being from 1950s nuclear tests, not it being primoridial or from space.
Michael & Sean, thanks for the clarification. Looks like my nuclear physics has gone a bit rusty over 20 years. Still sounds like the natural occurrence of anything "heavier" than Uranium on earth is likely to be very limited and very short-lived
Zoellner's comment is technically mistaken in the first sentence, although the error is minor. As a geologist I should point out that, strictly speaking, neither uranium nor anything in the periodic table are "minerals." Minerals are naturally occurring crystalline substances that are usually solid and usually inorganic. The periodic table contains elements. Uranium is therefore an 'element' not a 'mineral'. However uranium-bearing minerals ie. uraninite and coffinite are truly minerals and not elements. Sometimes people find the fact of variable compositions surprising, as it contradicts previous notions that all matter has a fixed chemical composition, ie. water is H2O, table salt is NaCl, etc. In mineralogy, nothing could be further from the truth. For example, the common mineral plagioclase exists as a solid solution varying from Na-rich albite to Ca-rich anorthite. Those two minerals are end-members, and do have specific compositions, but together create a series of compositions where both Na and Ca substitute for each other within the crystal lattice. Other minerals -- such sillimanite, kyanite, and andalusite -- all vary in appearance and properties, yet those 3 minerals all have exactly the same chemical formula, a phenomena referred to as polymorphism.
Why are comments made above so varied and confusing. Are the topics still being natal stage? Thanks.