"After all, the universe required ten billion years of evolution before life was even possible; the evolution of the stars and the evolving of new chemical elements in the nuclear furnaces of the stars were indispensable prerequisites for the generation of life." -John Polkinghorne
There are close to a whopping 1028 atoms in your body. And while just over half of them are hydrogen atoms, all the rest of them -- from Lithium to Uranium -- were made inside of stars, and ejected back out into the Universe, where, billions of years later, they made you.
In fact, a great many of these atoms didn't come from just anywhere, they came from a supernova! Last week, I told you what makes a supernova so super, and now it's time to tell you the story of just how this happens.
Our story begins all the way back when the earliest elements of the Universe -- Hydrogen and Helium -- were drawn together into massive clumps by the irresistible force of gravity, and formed the first stars.
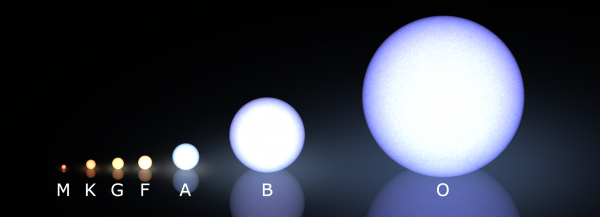
Whenever you form stars in this Universe, they come in a huge, wide range of masses. A few percent of the stars that we form are similar to our Sun, a G-type star, but the vast majority of stars are lower in mass, cooler, and redder in color. Somewhere between 90-95% of stars come in the two coolest, reddest varieties: K- and M-class stars.
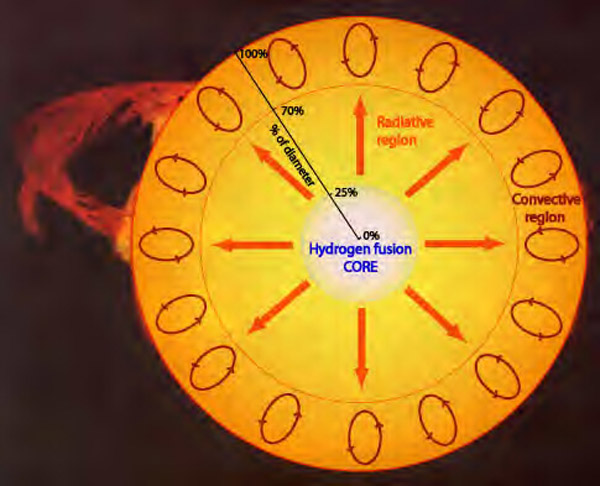
Regardless of what type of star you are, you get your energy from the same source: nuclear power! Overall, stars take the nucleus of four hydrogen atoms (which are just protons) and fuse them together to produce a helium nucleus, which contains two protons and two neutrons.
Now, I can hear some of you out there objecting to this. And it's a very clever objection.
Neutrons are more massive than protons, so how can you turn four protons into two protons and two neutrons?
Except you aren't making two free protons and two free neutrons, you are making one bound helium nucleus. And if you put four protons on one side of a scale, and one helium nucleus on the other side, you would find that the protons are heavier than the helium nucleus by a little bit: about 0.7%.
So as time goes on in your star, so long as the temperature in the core is hot enough, and there's enough hydrogen in there to fuse into helium, you keep on burning through your nuclear fuel. And that 0.7% mass difference between the hydrogen you started with and the helium you wind up with? That gets released as energy, from your old friend, E = mc2.
And all the stars, from the ultra-massive but equally rare O-stars (less than 0.1% of all stars!) to the numerous, tiny M-stars, burn their hydrogen into helium for their fuel.
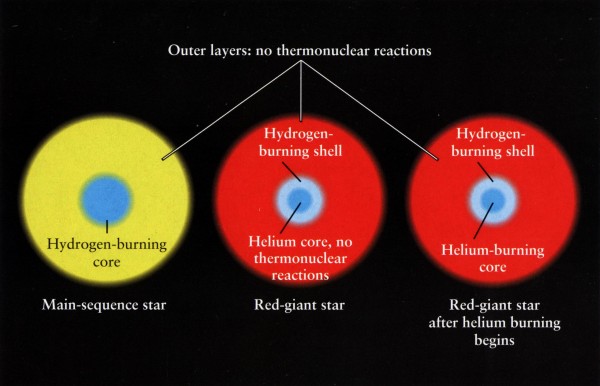
But they don't all do it at the same rate. The oldest M-star in the Universe has still not finished converting all of its hydrogen into helium, but an O-star can burn through all of the hydrogen in its core in under one million years. With the exception of M-stars, which will never be hot enough to reach the next stage, all the other star types -- including our own Sun a few billion years in the future -- will expand into a red giant, burning hydrogen in a shell around its helium core.
After a little while, the temperatures, pressures and densities inside the helium core become high enough that the helium atoms begin to fuse, turning every three helium atoms into a carbon atom.
Since carbon is more stable than helium, even more energy is given off via E = mc2. If you're a K-star, this is the end of the line for you. When you run out of helium fuel in your new core, you blow off your outer layers into a planetary nebula, while your core contracts over time, producing a white dwarf star that's comparable in mass to the Sun, but comparable in physical size to the Earth.
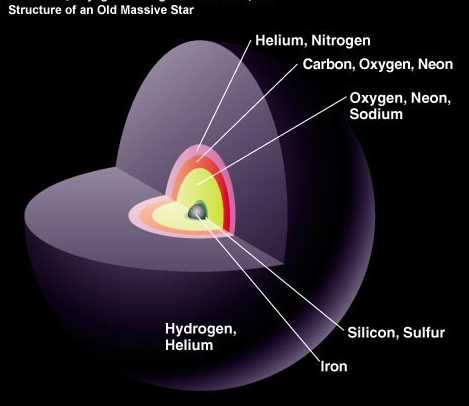
But the more massive stars continue to burn the heavier atoms into things like Oxygen and Neon, where the innermost layers fuse atoms into progressively heavier elements. But it's very hard to get elements that have high atomic numbers on the periodic table. Even a bright, massive, blue A-star will only fuse atoms up to Silicon and Sulfur, the fourteenth and sixteenth elements. (And only about 1% of stars are massive enough to be A-stars!) You can even be as heavy as one of the dimmer B-stars, and your fate will still be to blow off your outer layers in a planetary nebula, leaving behind an Earth-sized white dwarf that's comparable in mass, more-or-less, to the Sun.

But about one out of every 800 stars we make in this Universe is massive enough to go up beyond the element silicon, and will fuse elements all the way up to the heaviest ones we can make in stars: iron, nickel and cobalt.
It's kind of a misnomer to say that this is an "old" massive star; it may be less than 1% the current age of our Sun, which is still burning hydrogen in its core! But when the cores of these massive supergiant stars get large enough, the (mostly) iron atoms in there run into trouble.
Up until now, we've been fusing lighter elements into heavier ones, releasing energy with each successive step. But now, there's a tremendous pressure on the iron atoms at the core, but there's nowhere for them to go to give off more energy. Unless, of course, they implode.
And for stars more massive than about eight times our Sun, that's exactly what happens. There's a mass limit to the core -- about 1.38 times the mass of our Sun -- and when we reach or exceed that, the iron atoms at the core, themselves, collapse. The destruction of this huge number of atoms -- something like 1056 iron atoms -- releases a terrible amount of energy all at once! While the core collapses down to either a neutron star (something about the mass of the Sun but the size of a moderate asteroid) or a black hole, the outer layers get a rush of energy, the likes of which haven't been around since the earliest seconds of the Big Bang.
This release of energy not only blasts the outer layers across space for light-years, it makes possible the creation of all of the known elements of the periodic table. It doesn't merely make elements up to Uranium, either, but Plutonium, Curium and even higher mass, shorter-lived elements. The only reason Uranium and Plutonium are the heaviest naturally occurring elements on Earth is because the heavier atoms have had enough time that every single one has radioactively-decayed away.

Image credit: X-ray: NASA/CXC/Caltech/S.Kulkarni et al.; Optical: NASA/STScI/UIUC/Y.H.Chu & R.Williams et al.; IR: NASA/JPL-Caltech/R.Gehrz et al.
So when we see a star go supernova, we are witnessing the formation of all the elements we find on Earth that are heavier than iron. And this is the only place in the Universe where it happens!
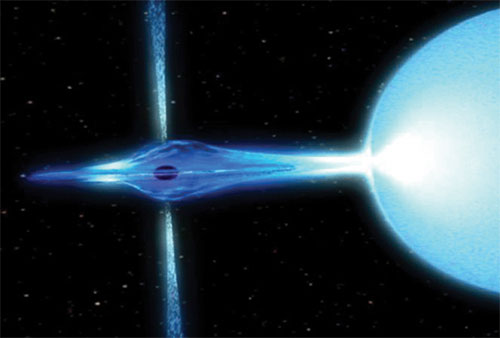
But just because you weren't born as one of those (lucky?) stars, those one-in-800 stars that have enough mass to form a type II supernova doesn't mean you'll never go supernova. On the contrary, stars that have burned their fuel and contracted to become white dwarf stars get a second chance!
White dwarf stars can either accrete material from a companion star, as shown above, or could merge with another white dwarf star, as shown below.
This is the other main type of supernova, a type Ia supernova. In this case, the runaway fusion reaction destroys the entire white dwarf, leaving no neutron star, black hole, or anything else behind!
And that's exactly what this star in the pinwheel galaxy did 21 million years ago!
If you've got clear skies in the early part of the night in the coming nights, now's the time to go and look for it.
And this process -- how stars die -- is how all the elements in the Universe that aren't hydrogen and helium are made. Not only that, but all the elements heavier than iron, including silver, gold, iodine, mercury, tin, lead, and uranium, had to have come from a supernova. As Carl Sagan said,
We are star stuff which has taken its destiny into its own hands.
Not just starstuff, in fact, but supernova stuff! This is the story of us all, simultaneously awe-inspiring and humbling to think of the generations of stars that lived, died, and recycled their elements, so that billions of years later, there was an Earth. This story is happening right now, in countless distant galaxies across the Universe. Don't miss your chance to glimpse the closest one in a generation.
(For my diligent and astute commenters.)







Bravo!
Awesome--thanks for posting that! Though I knew the difference in the formation of Type 1a and Type 2 supernovae, I didn't understand the mechanisms that clearly, especially the former. Great.
Looking at the wikipedia entry for Type II supernovae, I noted that over 40-50 solar masses, some suspect that the stellar core collapses so swiftly and completely that it doesn't even form a supernova--it goes straight to a black hole. WHOA. I wonder what that would look like--and if there are any candidates for such behavior in our neighborhood (Eta Carinae?). It sounds... impressive.
Ethan rules!
Great post - thanks!
Do you have an estimate on the ratio of hydrogen in our bodies that originated just after the big bang at the end of the ionization phase? I understand that free neutrons have protons as their decay detritus, but of the protons in our bodies, how many came from neutrons and how many from the original big bang? (or one could argue that we are made entirely from big bang left-overs because that's where everything came from, but I still like the idea of being "star-stuff" and a way for the universe to look in the mirror.)
"Do you have an estimate on the ratio of hydrogen in our bodies that originated just after the big bang at the end of the ionization phase?"
I have to ask: why? What will that tell you, even if it's possible to answer?
And, since your entire body only lasts a few years at the atomic level, being built up of new atoms over that time (absent some things like bones and so on), the idea of "your body" at this level is rather hard to pin down...
Never stop blogging please Ethan.
Where do black holes and neutron stars fit into all of this star exploding action? Couldn't you add a bit to the article to explain when they are created?
Ross:
You could argue that all the protons in our bodies came from neutrons. In the first minutes after the Big Bang, neutrons and protons were in thermal equilibrium, freely converting back and forth. Then the universe cooled enough to take this conversion reaction out of equilibrium, and within the next dozen minutes Big Bang Nucleosynthesis had locked up the free neutrons into (mostly) helium nuclei.
As for protons that came from neutrons produced after this time? I imagine that's a pretty small percentage, since neutrons are generally only produced by some nuclear reactions and cosmic ray events.
Cheers!
"leaving no neutron star, black hole, or anything else behind!"
There must be a stellar mass' worth of iron atoms floating around...
Forgive me if I got this wrong, but I thought all the lithium in the universe was from the Big Bang. Doesn't lithium get created, but almost immediately destroyed in stars?
Ok, this article is better. But still has several incorrect statements.
"And while just over half of them are hydrogen atoms, all the rest of them -- from Lithium to Uranium -- were made inside of stars, and ejected back out into the Universe, where, billions of years later, they made you. "
That's incorrect. Some amount of Lithium and Beryllium was formed during the Big Bang nucleosynthesis.
"So when we see a star go supernova, we are witnessing the formation of all the elements we find on Earth that are heavier than iron. And this is the only place in the Universe where it happens! "
That's WRONG! Elements heavier than iron are formed in normal stars with the help of s-process (basically, neutrons are captured by nuclei and then beta-decay to protons). That's why we can see Technetium in the spectrum of main-sequence stars.
Also an interesting fact: Silicon-burning phase of heavy stars lasts only several _days_. It takes so little time because most of fusion energy is carried away by neutrinos.
Alex B.,
You are of course right about the Lithium; the Lithium-7 in the Universe is predominantly from Big Bang Nucleosynthesis, and where the Lithium-6 originates from is still an open (and much-debated) question. The Beryllium produced in Big Bang Nucleosynthesis is expected to be non-zero, but is generally considered negligible compared to the amount observed in the Universe.
I knew about the three dredge-ups that happen in stars, but I did not know about the effectiveness of the s-process in building up the middle-of-the-periodic-table elements; I had never heard of Technetium stars but they do, in fact, exist! Thanks for that information!
My previous understanding of stars was that production of iron was energy-absorbing, and it was this fact that stopped stellar processes in their tracks. Am I wrong?
@Davem The production of iron is energy-releasing, but to produce anything *heavier* requires the input of energy, which is why fusion stops at iron.
It is my understanding that stars become red giants because helium is heavier than hydrogen, so thanks gravity it sinks into the center of the star. As lighter elements fuse into heavier elements, the heaviest ones fall into the center of the star and then there are lighter and lighter elements the further away from the center you get. In a K-star why is it that when there is no more helium fuel in the core the outer layers are blown away during a planetary nebula? How is it that only the outer layers of elements in the star are blown away, while the carbon core turns into a very dense white dwarf? My question really is why does the planetary nebula happen, and why can the white dwarf survive it?
"Don't miss your chance to glimpse the closest one in a generation."
One never knows when a statement like that will become retroactively pessimistic - Keep watching the skies!!!!!!
"The only reason Uranium and Plutonium are the heaviest naturally occurring elements on Earth is because the heavier atoms have had enough time that every single one has radioactively-decayed away. "
Are there estimates to the heaviest atoms created in supernovas? (no matter how shortly lived it may have been)
Most things in the world, as long as their inner parts are hidden, stand upright and live. If they are revealed, they die, as is illustrated by the visible man: as long as the intestines of the man are hidden, the man is alive; when his intestines are exposed and come out of him, the man will die. So also with the tree: while its root is hidden, it sprouts and grows. If its root is exposed, the tree dries up. So it is with every birth that is in the world, not only with the revealed but with the hidden. For so long as the root of wickedness is hidden, it is strong. But when it is recognized, it is dissolved. When it is revealed, it perishes. That is why the Word says, "Already the axe is laid at the root of the trees" (Mt 3:10). It will not merely cut - what is cut sprouts again - but the ax penetrates deeply, until it brings up the root. Jesus pulled out the root of the whole place, while others did it only partially. As for ourselves, let each one of us dig down after the root of evil which is within one, and let one pluck it out of one's heart from the root. It will be plucked out if we recognize it. But if we are ignorant of it, it takes root in us and produces its fruit in our heart. It masters us. We are its slaves. It takes us captive, to make us do what we do not want; and what we do want, we do not do. It is powerful because we have not recognized it. While it exists it is active. Ignorance is the mother of all evil. Ignorance will result in death, because those who come from ignorance neither were nor are nor shall be. [...] will be perfect when all the truth is revealed. For truth is like ignorance: while it is hidden, it rests in itself, but when it is revealed and is recognized, it is praised, inasmuch as it is stronger than ignorance and error. It gives freedom. The Word said, "If you know the truth, the truth will make you free" (Jn 8:32). Ignorance is a slave. Knowledge is freedom. If we know the truth, we shall find the fruits of the truth within us. If we are joined to it, it will bring our fulfillment.
Ludwig, we do not need stupid bible verses to ruin our understanding of things.
And that is precisely what this science is doing for us that those religious screeds never could.
I assume that's what you meant.