I have this one little saying. When things get too heavy just call me helium, the lightest known gas to man. -Jimi Hendrix
Hendrix is almost right: helium is the second lightest gas known to man, behind hydrogen. But there are many applications for helium -- both scientific and non-scientific -- that make it incredibly useful and practical. Helium is far lighter than air and is inert, which means it won't combust when you combine it with air and energy, like Hydrogen does (below).

(Too bad for the kids who want hydrogen balloons for their birthday parties!)
In addition to being lighter than air, Helium is incredibly useful, scientifically, in its liquid form! With a boiling point of only 4 Kelvin, liquid helium is used to cool some of the most powerful electromagnets on Earth, including those at Fermilab and the Large Hadron Collider. It's the first known superfluid, a fluid that has many interesting properties, including absolutely no viscosity, and it will never come to rest or lose energy once you start it in motion!

The exosphere -- the uppermost layer of the atmosphere -- contains small amounts of Helium. Compared to the rest of the atmosphere, five parts per million are Helium, which means that extracting Helium from the atmosphere is incredibly inefficient and expensive, so much so that we don't do it.
Where do we get our Helium from, then? Believe it or not, we mine it from underground!

This above image is the U.S. Bureau of Land Management's Crude Helium Enrichment Facility in Texas. It supplies about 40% of the Helium produced in my country, and it all comes from pockets of Helium that live underground.
Want to know how it got there?
That's right; radioactivity! You see, when our Earth was formed, it was loaded up with a whole slew of unstable elements, including everything on the periodic table that's heavier than Lead, such as Uranium, Thorium, Radium, and Radon. Since these elements are unstable, they radioactively decay.
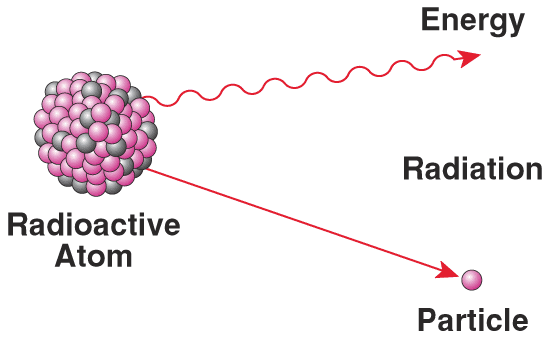
Even though some of these elements take billions of years to decay (on average), the Earth has been around for billions of years! Not only that, but there are three ways that particles can decay. The first type discovered -- alpha decay -- is when a radioactive particle emits a Helium nucleus!
Find a couple of electrons, and what have you got?
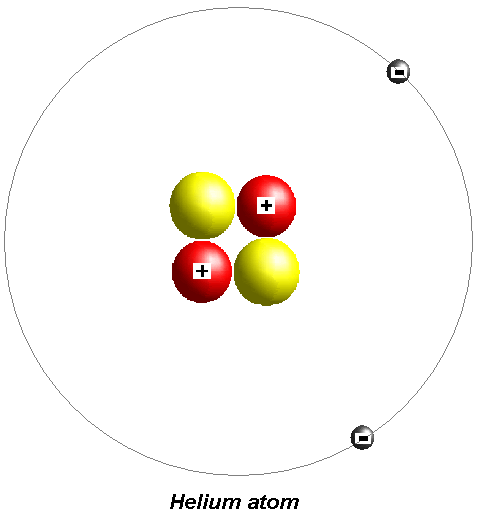
Helium! So if you get an ore of the right type of radioactive element, and you wait millions and millions (or even billions) of years, you make a giant underground store of helium!
So the good news is that these underground stores exist, so we have a supply of Helium for all of our scientific (and non-scientific) purposes. But the bad news? When we use it up, we'll have to wait million of years for it to build back up, or figure out some non-prohibitively expensive way to recover it from the atmosphere. (It's so expensive that many are thinking of mining the Moon when we're out of it on Earth!)
I'm all for environmentalism and conservation, but even if you aren't, running out of Helium poses a serious problem for the continuing advancement of science and technology. Helium on Earth is scarce. It took millions of years to make the stores we have now, and once they're gone, they'll be gone for thousands of generations.
So be careful with the precious things you have, and treat them like the precious things they are, even if others don't. Because some of them -- like Helium -- are truly irreplaceable.

Best Trekking agency in Kathmandu.
Trekking in Nepal
You never answer the main question of WHY Helium on earth is scarce. Is there a simple answer?
It's so light that it escapes from the atmosphere.
So.... no more helium balloons?
And, if helium is so scarce, why are helium balloons so common?
" ......it will never come to rest or lose energy once you start it in motion!"
Really? So why is this not powering turbines to produce electricity?
Great post again, thanks !
Well Stephen, Helium as a gas takes up a LOT more space than liquid Helium does, about 22.4 times at STP. So very little is actually required to fill a balloon compared to the rather large amounts required for cooling and scientific endeavours. Oh, and to fill them with the only reasonable alternative that would still allow them to rise in the air is potentially dangerous considering the age of your average helium balloon enthusiast : )
Hey, not so glum! We are, after all, working on ways to make more.
While the longer term problem is that demand is going to outstrip supply, the shorter term problem is that the supply is being mismanaged.
The government did a reasonable job, but the Republican ideologues wanted to privatize it, so the oil extractors are now in charge. Figure it out. Which pays better next quarter: maximizing oil extraction while wasting helium; or conserving long-term helium productivity potential at the expense of oil you can sell for $82 barrel?
That picture of the Hindenburg exploding shows kerosene fuel on fire, not hydrogen. Hydrogen oxidation radiates in the UV, and produces no soot. Of course, once the fuel tanks were blown up, the hydrogen went up too, but the H. would have been little better off if lofted with helium.
Small pedantic point re "when our Earth was formed, it was loaded up with a whole slew of unstable elements,including everything on the periodic table that's heavier than Lead, such as Uranium, Thorium, Radium, and Radon". Actually radium-226 (in uranium decay series) with a half-life of 1600 years and radon-222 with a half-life of 3.8 days are being continually replenished by decay of their parent uranium. The comment applies only to uranium and thorium.
People are thinking about mining the moon for He-3, which is a lot rarer than the usual He-4.
Of course if you consider isotopomers then Helium makes it to #3 (or a very close race with D2 between #3 and #4).
Mining the moon? What would the mechanism be for trapping He on the moon? Here on earth it tends to be trapped in the hydrocarbons (including natural gas to some extent) which are in turn trapped in ancient sand deposits with various minerals above acting as a seal (though seals aren't that crucial for the oil deposits where most of our helium comes from). The moon will have no significant hydrocarbon deposits because there was no life (or never abundant life) on the moon, so dissolution in hydrocarbons is out of the question. Since the moon has no rivers either and it is primarily prehistoric river systems on earth which create the storage and sealing formations, how do people imagine He will be trapped on the moon?
Unfortunately helium is not readily replaced in numerous applications since many applications do rely on the peculiar characteristics of helium. In many instances such as cooling detectors to very low temperatures we'll have to make do with far less efficient systems.
It's captured from the solar wind when it slams into the regolith.
There is some debate over what exactly is producing the visible flame in the Hindenburg footage, but I've never before heard the claim that it's kerosene. A more common claim (and one proven plausible by that geek instutition, Mythbusters) is that the flame and soot and sparks are the product of burning the doped muslin envelope, which, in the precise conditions of the Hindenburg, would act basically as thermite.
So I agree that the flame isn't entirely hydrogen (which burns nearly invisibly), but I disagree that it's kerosene. The envelope is clearly on fire; that's plenty of material to produce a colored flame.
When the helium from our balloons is let out does it go up and reside in the upper atmosphere or does it completely leave the earth?
In time the helium will completely leave earth. We're lucky that our planet has enough gravity to hold on to the nitrogen and oxygen we have in the atmosphere; Mars, being a bit smaller, lost those and all that's left is carbon dioxide.
Everyone,
Has annyone ever thought about FUSION to get all of this helium we dont have?? Hello!! I know Fusion might be 50, 100 even 1000 years away... but when its achieved in the right way there will be in theory an endless supply of elements(hopefully no supernova tho...lolz)
*J
I thought our main sources were Helium associated with natural gas. Of course it is fairly common for NG and Oil to be found together. So I think most of our supply came from a few formations where the natural gas (methane) had a high concentration of Helium. So in essence the Helium is a byproduct of some natural gas wells. The problem is the economics is driven by the fossil fuel, and not by some potential future high value of Helium. So standard accounting, which exponentially discounts the future, says "don't waste money capturing and storing more Helium than we need for current consumption". Of course the economics theory of substitutability, claims uh don't worry about not having any Helium in say 100 years, we will be clever enough to find an alternative doesn't recognize the unique properties of the stuff.
Mu. I think Mars loses CO2 as well. Its just that it has a reservoir of solid CO2 replenshing it. The earth as well loses some atmosphere, more so for the lighter molecules, but we do lose Oxygen, Nitrogen, and CO2 as well. Just not so much that the system has been depleted.
Something I have been wondering that is only tangentially related:
I am under the impression that the heaviest element that can be made by solar fusion is lead. Therefore, I am confused as to how all the heavier elements are made. Is my initial supposition wrong? If not, how do the elements heavier than Pb come into being?
Hi JBC,
Yep, your impression is incorrect. All the heavy elements are made in supernovas, including radioactive ones (radioactive decays power much of the visible display of the supernova). Lead's just the heaviest stable element.
Actually, iron is the heaviest element produced in normal fusion processes. The heavier elements were created only in supernovas of first and second generation stars.
Just a followup to #1 and #2:
#2 is correct that the helium evaporates from the earth. (Contrary to the implications of Ethan's article it won't stay in the exosphere forever. Calculating the fraction of the Boltzmann distribution that's above escape velocity is a fun physics problem.)
The other fact that explains why we're in danger of running out of helium and not hydrogen (which evaporates even faster!) is due to helium's inertness (as mentioned by Ethan). Most of our hydrogen gets bound up in heavier molecules with lower vapor pressures, but helium stands alone.
That's why helium recycling is important. If I don't recycle my aluminum can, the problem isn't that we lose the aluminum from the earth, it's just that it will take more energy to get those atoms back into a useful form than if I recycled it. If I don't recycle my helium (and my guess is most users don't), the atoms eventually leave the planet.
I was sitting with my kid at Chick-Fil-A a couple of years ago and she asked where helium came from for the balloons they had there. "From a compressed gas cylinder" was not an acceptable answer. So, I learned some of what you wrote on my own and got some great feedback from readers at this post:
http://scienceblogs.com/terrasig/2007/10/getting_a_rise_out_of_helium.p…
I learned that Qatar and Algeria are giving the US a run for our money in helium recovery. Josh Rosenau also pointed out that while some helium comes from Texas and is shipped from there, 2/3 comes from a site in SW Kansas via a pipeline to the Texas panhandle. Great stuff - I love learning all the things I blew off when I had freshman chemistry.
i don't know about the Hindenberg flames being due to kerosene. that stuff burns orange, and the Hindenberg is clearly burning in black and white. it must have been some super secret german gas.
i saw a special on PBS about how the fire started. it was always assumed that the hydrogen was ignited from a spark or lightning.
however, the special gave good evidence that the panels of doped cloth on the airship weren't properly grounded. they built up a large electrostatic potential between them which caused a spark that ignited the cloth itself. as the cloth burned, it ignited the hydrogen bladders, which didn't help the matter at all.
(they tracked down some samples of the doped cloth that was used on the airships and tested them for flammability by spark ignition)
Ethan
An off topic comment, related to your cosmic distance record blog and discussion of metal free stars.
You may not have seen this New Scientist article Primordial giant: The star that time forgot.
http://www.newscientist.com/article/mg20527470.900-primordial-giant-the…
It discusses a giant low metal star found in a nearby dwarf galaxy. A quote of interest, " this was a star that should not have existed, in a place where it should never have been. It was a mind-bogglingly massive star that was a throwback to a universe long since gone. "
OK that's a heads up for you.
Like yesterday, the video doesn't work for me. "An error occurred, please try again later." :-(
No. Mars (like, BTW, Venus) still has a lot of nitrogen, and never had any oxygen (except for traces produced by UV destroying water; those are still on Mars, though as part of the rust, not of the atmosphere).
What's going on is that the gravity of Mars is small enough (1/6 that of the Earth) that small asteroid impacts can erode the atmosphere. That seems to be what has happened, and why the atmosphere is so thin (the pressure is very low).
Wow, thanks a lot! Very impressive.
Standard disclaimer: I don't have a blog, I just need to pretend so I can comment on Pharyngula.
Am I the only one who read this and immediately thought of the episode of Gilligan's Island where they find the hole in the ground with helium flowing out of it? I hate to spoil it for those of you who didn't see it, but, sadly, their plan to get off the island in a helium balloon failed.
Bruce: i had forgot that one. but you reminded me of another episode where they discover a jungle boy living on the island. kinda like a young tarzan.
who was the actor that played the jungle boy?
oh, and to keep this on topic, at cosmicvariance i read that the LHC uses 96 tonnes of liquid He. TONNES! that would fill one heck of a party balloon.
Rob, the jungle boy episode actually was the helium episode. And the actor was... drum roll, please... Kurt Russell. Gilligan's Island and Big Trouble in Little China... now that's a resume!
@Dunc: So the helium nuclei hit the regolith - and then what? How is the helium trapped so that it doesn't simply find its way to the surface and into space? Like I said, here on earth it tends to be trapped by any number of layers which are effectively impermeable to He, and these layers tend to be associated with ancient marine or river systems.
This is a case where government policy makes all the difference. Helium is a constituent in most natural gas reservoirs--as you point out, it is alpha particles with captured electrons. It mixes with other gases and sits in the reservoirs at a low concentration. It makes sense for the government to subsidize the extraction of helium and make it available for scientific use as they did for many years. With the vast reserves of gas shales coming online, there will be loads of helium for the foreseeable future--if we extract it!
Another possibility in the long-term might be to capture helium from the solar wind, with a modified "solar sail". Alternatively, we might capture it from the atmosphere of either the sun itself, or one of the gas giants.
Are there any long term solutions being proposed or are we turning a blind eye on the situation and letting the next generation worry about it?
This was news to me - I wasn't aware there was a problem.
Why is helium so scarce on Earth? First, it has a very low molecular weight. Particles in a gas of uniform temperature roughly follow a particular probabilistic distribution of kinetic energies. A typical helium atom has one seventh the mass of an N2 molecule, so helium atoms jostle about at roughly the square root of seven times the speed of nitrogen molecules. This is too fast for Earth retain helium over geologic time periods. Second, it is a noble gas and does not bond chemically, like hydrogen, to form compounds or heavier molecules.
Nice article. One thing i still wonder is that we have technologically advanced so very much but unfortunately we can't 'create' an element from a different element. Guess its just the power of the nucleus that makes it so difficult.
One good thing about Helium is that since it is inert, it wouldn't chemically react with any other element/compound.
So basically all the used up Helium goes back into the 'exosphere' or 'stratosphere' whatever it is. Now how do we get it back from there? Is there a way to isolate it from air? As chemically it wouldn't be possible.
Neil from seo articles
@Neil Andrew:
Of course we can do that! Unfortunately, it requires lots of energy and time (and therefore lots of money)... See e. g. at Wikipedia: http://en.wikipedia.org/wiki/Nuclear_transmutation
While helium occurs in minute quantities, that we are mining it faster than it is able to regenerate itself is clear testimony that we are living unsustainably. And in many ways, helium is a microcosm of the many of the natural resources such as fish, oil and forests. This, for me, is a far greater problem than the fact that the absence of helium will slow the advancement of science and technology.
As is the case in most situations, am sure the scientific fraternity will come up with a substitute of some sort or even some synthetic helium. Governments, universities and corporations would therefore be well-advised to channel R&D resources to finding a solution to this problem. Seems to me it will be quite lucrative.
I always thought that balloons were made of helium, at least the ones that float. I love the way your voice changes!
The way our atmosphere is being polluted and the ozone layer getting thinner, the only stable resource we have for now is our land.
Whatever scientific research is using helium, are they really necessary? And since our helium source is really low, shouldn't we try to find other alternatives before it actually runs out & we would have to suffer more consequences because of that? We should try not to keep sucking our resources dry every time! That's the very reason we have such a long list of endangered species and natural disasters!
Craigslist leads all other online classified ad sites and holds the number one position year after year.
It pulls millions of prospective customers every month worldwide.
You have the ability to submit ads for whatever you want.
. If you currently use Craigslist, or plan on using Craigslist as a business to make money.
just visit:
http://www.cladgenius.com
Guess its just the power of the nucleus that makes it so difficult.
Why is helium so scarce? I never realized that there was helium underground as well!
Is Helium non-renewable. I don't see how it can scarce if it is still so cheap. Maybe just a little too early to tell.
Helium on Earth is scarce. It took millions of years to make the stores we have now, and once they're gone, they'll be gone for thousands of generations.
Never knew that Helium can be very dangerous ! OMG
Best
http://www.babapandey.com/
Why can't humans just leave this stuff alone? If we use it all we can't use it for further research or better purposses just like oil. We use it all and then we move on, no idea what the consequences are. But who cares where our children have to live, we lived good.
Helium in the wrong hands is dangerous, people are just destroying our resources with little thought to what they are doin got our planet or the legacy we are leaving the next generation.Noosa accommodation
It took millions of years to make the stores we have now, and once they're gone, they'll be gone for thousands of generations.
Because some of them -- like Helium -- are truly irreplaceable.
âI am a possibilist. I believe that humanity is master of its own fate... Before we can change direction, we have to question many of the assumptions underlying our current philosophy. Assumptions like bigger is better; you can't stop progress; no speed is too fast; globalization is good.
Windows Defender
Helium is very light and as far as I know it is lighter than hydrogen (I think). It is one of the most useful applications that most people can make use of. It is really great.
I was unaware that helium was that scarce on planet earth. I figured it to be a common element and thus it would be found all over the atmosphere...I guess not.
If hydrogen is lighter than helium and helium is rare because it is so light then why is hydrogen not rare also?
We only have 16 helium mines in the world and they will not last long. When we are using the helium for balloons then it will escape into the atmosphere and we will not get it back again.
Helium is rare because it is't as reactive as hydrogen. Hydrogen is very good to make bonding to other atoms.
Why can't we just leave it alone? If we use it all we can't use it for further research or better purposes just like oil. We use it all and then we move on, no idea what the consequences are. But who cares where our children have to live, we lived good.
The problem is the economics is driven by the fossil fuel, and not by some potential future high value of Helium. So standard accounting, which exponentially discounts the future, says "don't waste money capturing and storing more Helium than we need for current consumption". Of course the economics theory of substitutability, claims uh don't worry about not having any Helium in say 100 years, we will be clever enough to find an alternative doesn't recognize the unique properties of the stuff. How To Get Abs.
Of course you don't store something like helium until it becomes commercially viable. Once the viability exists so will the means to produce/capture it. And we may never use it.
Lisa Krempasky
You see if we use it all we can't use it for further research or better purposses just like oil. We use it all and then we move on, no idea what the consequences are. But who cares where our children have to live, we lived good. regards pc software thanks.
It's the first known super-fluid, a fluid that has many interesting properties, including absolutely no viscosity, and it will never come to rest or lose energy once you start it in motion!
What a fascinating article! I didn't know any of that info about Helium that you posted. Where else is it mined besides Texas? It must be very expensive if it takes billions of years to produce, right?
Yet, there always seems to be no lack of helium at the grocery store for those who want to buy helium balloons-you know, those mylar things filled with helium. I've even seen large tanks full of the stuff at children's birthday parties.
Are we wasting helium or is there plenty of it to go around?
As a houston locksmith with an inquiring mind, I would like to know the answers.
Helium is incredibly useful, scientifically, in its liquid form! With a boiling point of only 4 Kelvin, liquid helium is used to cool some of the most powerful electromagnets on Earth, including those at Fermilab and the Large Hadron Collider.
last post :http://iwantedyoulyrics.blogspot.com
Helium on Earth is scarce. It took millions of years to make the stores we have now, and once they're gone, they'll be gone for thousands of generations.
Science and Honor - that is 22.4 liters per mole (not times)
I was also not aware at all that helium is so rare. Who is actually in control of most of the earth´s helium reserves?
Scientists say that Earth's helium reserves will run outn within 25 - 30 years.
This will be a dsaster for hospitals which use it to cool MRI scanners.
Whatever will happen to our kids balloons if Helium never existed?? haha:) I'm so glad I have my canon lenses to capture my daughter's smile.:)
o nice pic.
You see if we use it all we can't use it for further research or better purposses just like oil. We use it all and then we move on, no idea what the consequences are. But who cares where our children have to live, we lived good. regards pc software thanks.
So if Helium is becoming very rare on Earth, and we are running out of it, with Helium being the second most common element in the Universe (behind Hydrogen) does that mean it is theoretically possible for the planet to use up all the helium it has? If this was the case what would the result be?
Would that scenario ever be possible?
Matched Betting
Just curious
First of all, you blew my mind with all of that "superfluid" stuff. I never knew that Helium was so versatile. Much like half the people commenting on this post, I thought helium was just a gas that kept our balloons afloat. I mean, I knew that it was used to cool things like the Large Hadron Collider(I figure it takes a little more than ice from a manitowoc ice machine to cool something like that), but a superfuid with absolutely no viscosity? Imagine my surprise, eh? After watching that video though, I see helium is some serious stuff. Secondly, you mentioned that it is very expensive to scavenge helium out of the atmosphere, so some people are considering mining the moon for it. I am not a scientist, so bear with me . . .how do we know for certain that the moon has the same helium stores that earth does? Also, how in the world can that option be any less expensive than getting it from our own atmosphere. Just a couple of thoughts. Thanks again for the great information.
First of all, i didnt knew that helium can be in liquid state. This makes me think if helium could be used in cars , as a fuel...
Thanks as I never knew helium came from mining underground. Also, the pockets you speak of quite possibly could be blamed for the BP oil spill, no? SEO Optimisation
Thanks as I never knew helium came from mining underground. Also, the pockets you speak of quite possibly could be blamed for the BP oil spill, no? SEO Optimisation
helium is so light that it escapes from earth but why dont the hydrogen lighter than helium escape from earth???
Thanks very much for the post i really didnt know there was so much information on helium! Really enjoyed the read! thanks again
Why can't we just leave it alone? If we use it all we can't use it for further research or better purposes just like oil. We use it all and then we move on, no idea what the consequences are. But who cares where our children have to live, we lived good.
Helium can be stable in specific quantities. I wouldn't feel safe into that kind of balloon-plane though ;] But who would?
Heck, build something safe and then people will board. But I won't risk my life beforehand if I'm not 99.99% sure I'm safe
I love the way you make things look simple and funny at the same time. I'm by no means a scientist but I read your post from top to bottom and I did get more interested in the applications of Helium and how we're going to deal with things when we're out of it on earth. Thanks for this entertaining article. Hank Reiss from repossessed cars information
I am not a scientist, so bear with me . . .how do we know for certain that the moon has the same helium stores that earth does? Also, how in the world can that option be any less expensive than getting it from our own atmosphere. life experience degrees
Helium on earth is really scarce news for us. I watch the video and now i know why is so scarce.
Why can't we just leave it alone? If we use it all we can't use it for further research or better purposes just like oil. We use it all and then we move on, no idea what the consequences are.
The video is quite interesting for those into physics. I believe it's taken from the BBC documentary "Absolute zero". Still don't understand though, why such shortage on earth. I truly believed we had it in large quantities, such as the other gases. Could it be too soon to tell?
Sandro from Italian Pop Music
"So.... no more helium balloons?
And, if helium is so scarce, why are helium balloons so common?"
Opportunity costs are not accounted for in the helium-balloon production process.
Charlie Lyne is the "12-yr old" co-presenter on the show. But Winkleman got her words a bit mixed up when saying it - the unexpected "I love Charlie, I do" just seemed like an unabashed admission of cocaine abuse!
trading
Helium is an inert or noble gas from Group 18 of the periodic table. In general, it does not want to bond with anything else. Provillus It exists as a monatomic gas floating around in single-atom
Yeah!, The amount of helium in a tank depends on how big the tank is, the pressure of the helium and its temperature. It is calculated from the ideal gas equation with allowance for compressibility.
Nowadays there are so many threats to our planet, that's why we should do our best to save it.
First of all, i didnt knew that helium can be in liquid state. This makes me think if helium could be used in cars. Mind you if scarcity is such an issue then it mighjt not be an effective use of resources? http://www.onlineforextradingstrategy.com there's got to be an effective method of manufacturing it though doesn't there? seems that there's a way to mass produce just about everything else nowadays.
if we run out of helium. there's going to be a lot of baloon vendors heading onto welfare.... http://www.mortgagelasvegasnevada.com/bad-credit-mortgages.php
given the amount of gas used in the hindenberg, and it's catasprohic demise, I think we'd all be wise to look at helium as a resource that should be safeguarded rather than squandered http://www.floridarefinancemortgagerates.com it does seem strange though that it's so much scarcer than other gasses though
it's not an industrially used gas, so it shouldn't be that big of a deal to most of us, but it's probably critical not to run out or even come close http://www.betterseoservices.com as then you really find out how much it does get used in small quantities.
when it comes to applications, a gas that's lighter than the general atmosphere is always going to be handy...and lets not forget the voice-raising side effetcs at parties! http://www.mortgagerefinancelowrate.net gotta save the helium - children around the world demand it!
sneaky stuff that helium - i had it pumped up my nose while asleep after a kegger - freaked the heck out of me when I woke up at tried to yell at the a-hole that did it to me! http://www.mortgagebaltimoremaryland.com
as a resource, there are worse things to be threatened than a lack of helium, but it's still never a good look.
Thanks - I never knew helium came from mining underground. sseems like it should be the result of a chemical reaction I don't know why exactly i think thats the case, but it just seems to make sense to me for some reason. silly i know
Maybe I've got this completely wrong, but wouldn't photon-photon collisions produce matter/antimatter pairs, not simply matter particles? I think we're running in to an ambiguity of wording between matter as distinct from energy and matter as opposed to antimatter.www.itunes.com
kvien,
I think the terms are just fine but perhaps your understanding of the subject matter is imperfect aluminum cases? you should also check this out:
"Charlie Lyne is the "12-yr old" co-presenter on the show. But Winkleman got her words a bit mixed up when saying it - the unexpected "I love Charlie, I do" just seemed like an unabashed admission of cocaine abuse!"
as far as i can see this bears no relevance to anythig at all.
Hendrix? How did the original god of guitar get involved in this debate? Jimi was many things but a scientist he was not!
First of all, i didnt knew that helium can be in liquid state. This makes me think if helium could be used in cars. Mind you if scarcity is such an issue then it mighjt not be an effective use of resources http://www.mortgagelasvegasnevada.comthere's got to be an effective method of manufacturing it or at least replicating it.
Hey nick - not there doesn't have to be a way or replicating it, there are plenty of things we cant synthesize, gold for example and many, many other elements. when the http://www.mortgagelasvegasnevada.com helium is gone though it must still remain in the atmosphere, so perhaps it can be recaptured or trapped in some way??
being liquid and colourless at room temperature does make it tough, but there has to be a way or capturing helium gas once it's released? http://www.mortgagedetroitmichigan.com I'd certainly hope so at any rate as otherwise a large concentration of helium in the atmosphere could see us all talking like cartoon characters
Veee! that VHS tape of Steve Martin Wild & Crazy Guy, if you really watch it, when Steve is playing a straight song on the banjo you can see his entire http://www.thetrendystyle.com face, and demeanor change from the rest of the act. Completely different body language.
At that time i was also in london and attend this festival. that was first The project uses AudioBoo on the iPhone or iTouch, to enter content to the project, you simply Tag a Boo with âaudioboothâ
Well, digging out from underground is not what i was expecting. Really...But your point as to "why it cannot be sucked out from atmosphere" explains and elaborates everything.
Ethan, my man! You make the science all the more interesting. ;)
But you are showing pic of Hindenburg disaster just coz it was filled with hydrogen. Are you forgetting a bigger casualty disaster "USS Akron". I would like mention that USS Akron was filled with helium and nearly all the passengers died.
And Helium is not scarce :p
Next to hydrogen, it is the second most abundant element in the universe, and accounts for 24% of the elemental mass of our galaxy. So how can it be scarce???
Helium it is not what one would consider a renewable resource as it is lost once released to the atmosphere therefore the government stepped in and created a program to recover helium from natural gas and store the surplus.
Helium in a tank depends on how big the tank is, the pressure of the helium. http://www.oncanadameds.com/ And its temperature.
Many elements of fashion here, but composed rather poorly. Is this all it takes to get my picture taken in Paris? Ironically, I like the sweatpants best.
Helium is very important for us, a lot things we can do. The supplies we have on Earth come from radioactive alpha decay in rocks. Right now itâs not commercially viable to recover helium from the air, so we have to rely on extracting it from rocks.
I'm by no means a scientist but I read your post from top to bottom and I did get more interested in the applications of Helium
Maybe I've got this completely wrong, but wouldn't photon-photon collisions produce matter/antimatter pairs, not simply matter particles?
Very interesting post, extemely well thought out and put together. Keep up the good work.
Hi. I think that if more people thought about it that way, theyâd have a better time understanding the issue.
A lot of interesting facts about helium. You'd think if there was some kind of shortage we'd be seeing a lot less balloons around. It seems like a waste of a useful resource. Then again, humans are a very wasteful species.
I didn't know helium was such so useful.
I always thought it was used to make things float (lighter then air) not to help cool magnets in water.
Very useful information. I appreciate your effort, very well written article. I believe it's taken from the BBC documentary "Absolute zero". Still don't understand though, why such shortage on earth. I truly believed we had it in large quantities, such as the other gases. Could it be too soon to tell?
Exam Questions
More linkspam from Naresh above.
BBC documentary "Absolute zero". Still don't understand though, why such shortage on earth. I truly believed we had it in large quantities, such as the other gases. Could it be too soon to tell?
I admire what you have done here. I love the part where you say you are doing this to give back but I would assume by all the comments that is working for you as well. Do you have any more info on this?
I think it is fascinating that helium reacts that was, especially after you read how Feynman formulated his theories on it.
in my opinion it is not use on "full" power. I guess that there are many other domains where helium can be used.
Because people can not leave this stuff alone? If we use all that we can use for further studies or better purposses like oil. We use it all and then go ahead, do not know what the consequences. But who cares where our children are forced to live, we lived well.
Helium in the wrong hands is dangerous, people are just destroying our resources with little thought to what they are doin got our planet or the legacy we are leaving the next generation.
This was really helpful. Thank you for posting this! I am wondering why they made the program so hard to use if it requires that much work?
This thread is infested with spambots. In fact the only one that might not be a linkspam goes all the way back to 107. 20 continuous spambots.
I'm gonna stock up on helium. You never know when you might need some.
Seriously, Hendrix was wrong. Helium is the 3rd lightest gas in the universe, right behind Hydrogen and the stuff between the ears of lunatic liberals.
Its a shame that this post has been attacked by spam because some interesting points have been made.
It feels really good to know about the mysteries of scarcity of helium gas.Thanks for detailed posting about this gas, it's importance, usage, and threats of abusing or over using of this valuable gas.This inert gas is as light as Hydrogen gas, and it's atom is in second position on the periodic table.
What a nice article that i'm sure students get ideas from it.
I read from somewhere that First, Helium does not chemically combine with other elements.
Second, Helium is a very light weight (low density) gas that tends to rise in the atmosphere and eventually escape from the gravitational field of the Earth.
Last, there are very few sources for the production of Helium. Some radioactive elements release Alpha particles which eventually become Helium atoms after capturing electrons.
Read more: http://wiki.answers.com/Q/Why_is_helium_so_rare_on_earth#ixzz1VwOppNyP
Is it dangerous to inhale helium? I remember when I was a child, we used to breathe the helium from balloons and then talk like Daffy Duck and laugh our foolish heads off.
Anyone else do this too?
Indeed, it is nice compilation and I will use this one in my presentation. I am really like the way it was written. I am also thankful that I was able to check this site.
Indeed, it is nice article and I will use this one in my documentation. I am really like the way it was written. I am also thankful that I was able to find this site.
Because people can not leave this stuff alone? If we use all that we can use for further studies or better purposses like oil. We use it all and then go ahead, do not know what the consequences. But who cares where our children are forced to live, we lived well.
And to think i was gonna try and build myself a private airship. :l well this certainly put me out. :l
Because people can not leave this stuff alone? If we use all that we can use for further studies or better purposses like oil. We use it all and then go ahead, do not know what the consequences. But who cares where our children are forced to live, we lived well.
Not sure why helium is so scarce. Maybe we have used so much of it already? We need to find a cure for colon cancer though.
http://www.getscreenedtoday.com/
The reason the atmosphere thinned so much on Mars was because it's core cooled far quicker than ours is, and it lost its protective magnetosphere which protects our atmosphere from blowing away by solar winds.
Seems like the market isn't working? Shouldn't helium be more expensive? Much too expensive to be frivolously wasted in party balloons and 'hear me talk funny'?
The reason helium balloons are so common is that it is being sold at prices that don't reflect it's scarcity, due to subsidies. We should stop that! Helium is needed for important medical and scientific purposes, and we should stop wasting them on balloons immediately!
The reason why helium balloons are so common is due to two reasons. one being due to it subsidies but also the helium that we use in every day balloons is the gaseous version of it which equates to a small amout of what if being mined. Helium being used for medical and scientific use is in liquid form for the most part and it gets used in larger quantities. Helium is finite and we will run short in the next 30-50 years maybe sooner, however we have found ways to produce a gas equivelent to helium however the process is protected by patents just like we can get 100 miles to the gallon in a 1.8L engine however its not monitarily in the best intrest of the large companies that profit from selling it. Just as a side note, if you want to be well off in the near future, invest in assets such as helium, clean water and Virgin Galactic. Thats where my money goes.
Not only does helium escape Earth into space, it will also leave the solar system as the solar wind pushes it into interstellar space. The ultimate escape artist... every time you pop a helium balloon, you send something to the stars!
Very Nice.
Thanks for the posting . Can anyone tell me how much 'feedstock' [ ie radioactive material] was originally needed to produce the He that was found in one of those mines , and how that compares to the remaining elements that caused it. ?
Helium probably gets blown off the top of the atmosphere by solar winds. Hydrogen probably does also.
Helium is probably made by fusion of Hydrogen. 4 Hydrogen protons fuse to a Helium of 2 proton and 2 neutron.
Thermonuclear bombs fuse Hydrogen to Helium but catching helium from a bomb detonation is absurd. Maybe some day someone will learn to create a small hydrogen fusion to generate and trap Helium. Neutron bombardment may not cut it. Phase synced lasers are now known to generate particles. Perhaps someone will figure out an efficient small-scale method of fusing Hydrogen to become Helium and capture both the energy and Helium to use.
I guess the question then becomes "Where will people get more water (Hydrogen) to make Helium?".
We can break down the properties of helium and mend new artifical helium same as 12 carbons with oxygen