Pharmaceutical Law
In our last post, we discussed a recent USPTO ruling that rejected a claim of the Pfizer patent on the erectile dysfunction drug, sildenafil (Viagra®), to a novel oral treatment for the disorder. The patent appeals panel ruled that the existence of horny goat weed, a traditional Chinese medicine used orally for impotence, was grounds for rejection of the claim.
Frequent commenter daedalus4u pulled out US Patent #6,469,012 (filed Mar 4 1996, issued Oct 22 2002) wherein the relevant claim 24 reads:
A method of treating erectile dysfunction in a male human, comprising orally administering to a…
Reuters and Bloomberg reported earlier this week on an ongoing patent battle (read: pissing match) between Pfizer and Eli Lilly & Co. relating to their erectile dysfunction drugs Viagra and Cialis, respectively.
A US Patent and Trademark Office (USPTO) appeals committee has ruled that an element of Pfizer's patent on sildenafil, the active chemical in Viagra, is invalid because the drug is insufficiently different from a traditional Chinese medicine called Yin Yang Huo or horny goat weed.
At issue is Pfizer's claim to a method for treating male erectile dysfunction. The patent appeals…
It seems that bodybuilding supplement makers are challenging erectile dysfunction supplement makers to see who can recall the greatest number of products adulterated with undeclared, unapproved drugs.
In this case, an internet retailer of the following supplements has issued a voluntary recall of the following supplements sold between June 1, 2009 and November 17, 2009. The recall follows an FDA warning letter on detection of undeclared, synthetic anabolic steroids in these products:
Advanced Muscle Science Dienedrone, 60 caps
Advanced Muscle Science Liquidrone, 60 ml
Anabolic Formulation M1…
In an article by Wayne Heilman of the Colorado Springs Gazette, I learned of a US FDA action against contract drug manufacturer Provident Pharmaceuticals. The company has been cited for good manufacturing practices (GMP) violations as well as the manufacture of unapproved drugs. The GMP violations are of a magnitude that FDA requested that Provident hire a GMP training consultant to bring the facility up to snuff.
However, Heilman seized upon an interesting side light in the warning: the names of the unapproved drugs apparently made by the company are missing from the detailed warning letter…
By most metrics, those of us at Terra Sig World Headquarters are liberals. Nevertheless, we often enjoy reading conservative writer, attorney, and American Enterprise Institute fellow, David Frum. Perhaps I have a soft spot for him because he's Canadian and he also writes for my favorite print newsmagazine, The Week.
Well, Frum chose this week to write, "Herbal remedies need real scrutiny," at his FrumForum and the post was subsequently published as a special commentary at CNN.com. The latter version has accumulated about ten times as many comments. The thesis of his essay is that the…
In September we posted "M.D. Anderson name misused in Evolv nutraceutical water advertising," detailing the not-exactly-truthful claim by a multilevel marketing company that their bottled water product was "tested" by one of North America's premier teaching and research hospitals.
A flurry of search engine hits to this post raised my attention to the fact that the The University of Texas M.D. Anderson Cancer Center has now initiated legal action against the makers of Evolv. Cameron Langford at Courthouse News Service reports:
Two companies are pushing bottled tap water with false claims…
I should probably create a new blogpost category just for erectile dysfunction dietary supplements adulterated with authentic or synthetic analogs of prescription phosphodiesterase-5 (PDE5) inhibitors (e.g., Viagra, Cialis).
However, FDA has already created a page for this earlier this year after dozens of companies have been identified as putting real drugs into their erectile dysfunction products.
Do the brains behind these companies not realize that FDA is now monitoring every erectile dysfunction supplement for all manner of PDE5 inhibitors?
Apparently not:
For Immediate Release: Nov. 5…
Rebecca Skloot, journalist, University of Memphis writing professor, and author of the upcoming book, The Immortal Life of Henrietta Lacks (pre-order here), just brought to my attention this commentary by Dr Pieter Cohen in the New England Journal of Medicine entitled, "American Roulette -- Contaminated Dietary Supplements."
In the commentary, Dr Cohen remarks upon the epidemic of adulteration of herbal and non-herbal dietary supplements with undeclared prescription drugs or unapproved drugs:
In July 2009, the FDA expanded its alert to include 75 tainted weight-loss products that contain…
We've spoken here every few months about so-called natural dietary supplements being adulterated with prescription drugs used for similar indications. The most common of these of late have been erectile dysfunction supplements which have been repeatedly found to contain the active compounds present in prescription E.D. products such as Viagra and Cialis.
The latest public health advisory from the US FDA concerns what appears to be a much more serious case of adulterations, this time with steroids in body-building supplements marketed as containing "steroid-like" compounds:
The FDA is…
Salvia divinorum (Salvia, Magic Mint) is a plant used for entheogenic purposes by the Mazatec people of Mexico. A relative of the common garden plant "scarlet sage" (Salvia splendens), S. divinorum contains several hallucinogens that include salvinorin A, the first non-nitrogenous agonist known for kappa opioid receptors (KOR).
I had known of salvinorin A since a highly-cited 2002 Proceedings of the National Academy of Sciences paper by Bryan Roth, Richard Rothman and colleagues (full text here). At that time, I had read several anecdotal reports (that I cannot locate now) that the…
FDA and the New York Times are reporting today 23 cases of adverse reactions to various Hydroxycut weight loss supplements. (FDA Consumer PDF here).
Above and beyond whether the stuff actually helps with weight loss, it is clear that the products contain some compound(s) that cause idiosyncratic cases of liver damage.
According to the law governing dietary supplements, the F.D.A. is empowered to act only in cases when it identifies a harmful or adulterated product that is already on sale.
"Part of the problem as you know is that F.D.A. looks at dietary supplements from a post-market…
Regular commenter and friend of Terra Sig, leigh (the path forward), let us know overnight that Florida State Veterinarian Dr Thomas J Holt officially confirmed that selenium overdose killed 21 Venezuelian polo horses in Wellington, Florida, on 19 April.
An attempt by an Ocala, Florida, compounding pharmacy to reproduce an equine dietary supplement called Biodyl resulted in a toxic dose of sodium selenite to be given to the horses. The pharmacy is cooperating fully with the FDA and other authorities but there is no official word as to whether the pharmacy made a calculation error or were…
Last weekend, 21 Venezuelan polo horses collapsed and died at the US Open championship match at the International Polo Club Palm Beach in Wellington, Florida (AP, CNN). The deaths have now been associated with injection of a veterinary mineral supplement produced by a compounding pharmacy in Ocala, Florida. Located in central Florida about 45 min south of the University of Florida, Ocala is well-known for its density of equestrian farms and training centers.
Precisely how this supplement killed the animals is not yet known but I can guarantee that it was a calculation error involving an…
Back in February 2007, we had a lively discussion on a post about pharmacist objection to filling prescriptions for drugs they felt went again their personal moral stance: from morning-after pills to garden-variety oral contraceptives. I held that since pharmacists are licensed by the state to provide a service to the citizenry (and they make about as much money as a full professor at a US research university), they should be required to fill prescriptions. Some commenters argued that ethical practice of one's profession dictate that one apply moral standards. I disagree. As an agent of…
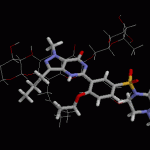
No, it's not a song by Foreigner - these are the names of two products "promoted and sold over the Internet for the treatment of erectile dysfunction (ED) and for sexual enhancement."
In yet another instance of a trend that would be comical if not so serious, the US FDA has announced that "Blue Steel" and "Hero" supplements contain chemical relatives of sildenafil, the active constituent of the prescription medication Viagra.
"Because these products are labeled as 'all natural dietary supplements,' consumers may assume that they are harmless and pose no health risk," said Janet Woodcock, M.…
Last week we spent some time discussing the shortcomings of the generic vs. brand name drug debate, focusing on an example of non-bioequivalence between the antidepressant Wellbutrin XL and its generic competitors.
Three days later, I then received an e-mail from one John Procter about a movement to get Washington to move forward on the approval of lower-priced generic biotechnology drugs now that original branded products are facing patent expiration. One source indicates that a $20 billion market value of biological products will be coming off patent by 2015. The US FDA has been reluctant…
I wrote about the general issue of eyelash-enriching "cosmeceuticals" on my Terra Sigillata back in July. Increasing the number and thickness of eyelashes is not some hokey magical wish - it is a known side effect buried in the prescribing information for the prescription glaucoma drugs, bimatoprost (Lumigan®) and latanoprost (Xalatan®).
Last Friday, the US FDA seized a product called Age Intervention Eyelash at the San Jose facility of Jan Marini Skin Research, Inc., citing that the product contained an unapproved drug and could be harmful to the user's vision.
When composing my post in…
Just one last comment on the recently passed FDA legislation. I know that Terra Sig readers must be tiring of this issue already, but this aspect was too good to pass up. I started writing this post on a lark but the topic actually has serious public health implications.
John Mack at his Pharma Marketing Blog made the clever observation that while DTC restrictions were not in the Senate bill, a provision "prohibiting the FDA from restricting the sale of turtles less than 10.2 centimeters in diameter as a pet DID make it into the bill (Title VII - Domestic Pet Turtle Market Access; Section…
The Pump Handle's Liz Borowski put up a nice post summarizing the key points of the >400-page Food and Drug Administration Amendments Act of 2007 (H.R. 3580).
Missing from the bill were any further restrictions on pharmaceutical direct-to-consumer (DTC) drug advertising - according to Liz, some drug safety advocates were calling for a complete ban on DTC ads.
Since the FDA began permitting DTC advertising in 1997, the purpose of the ads has been viewed as less about patient education for underdiagnosed diseases (the original pitch) and more about getting patients to request specific…