"Ours is a world of nuclear giants and ethical infants. We know more about war that we know about peace, more about killing that we know about living." -Omar N. Bradley
Nuclear physics is one of the most daunting, emotionally charged phrases in all of science. You can hardly say the words without the image of a mushroom cloud popping into most people's heads, followed by the devastations of radiation sickness and lingering radioactivity.
But -- as a physicist -- that's not what I think of at all.
Think down to all the basic constituents of matter, down beneath your cells, your organelles, the molecules that make them up all the way down to the individual atoms that make up the elements of everything on Earth.
 Image credit: CC 3.0, via https://grade-56g.wikispaces.com/.
Image credit: CC 3.0, via https://grade-56g.wikispaces.com/.
At the heart of every atom is an atomic nucleus made up of some combination of protons and neutrons, which in turn are made up of even more fundamental particles known as quarks and gluons. When I think of nuclear physics, I think of tremendous numbers of these little guys -- Avogadro's Number's worth of them -- and how they combine together on the smallest scales.
At the most fundamental level (that we know of), the quarks and gluons bind together, with three quarks making up each and every nucleon, where a nucleon is a general term for either a proton or a neutron.
Neutrons and protons aren't just made up of three quarks apiece, though, and you might have guessed that if you looked up the masses of the quarks and the masses of protons and neutrons.
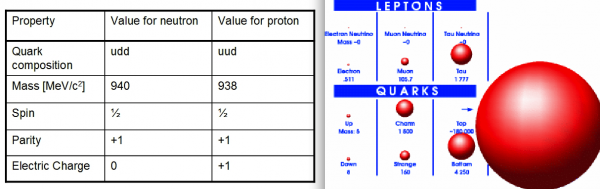 Image credit: http://cronodon.com/ (L), Fermilab / D0 (R).
Image credit: http://cronodon.com/ (L), Fermilab / D0 (R).
We typically say that a proton is made up of two up and one down quark, and that a neutron is made up of one up and two downs. But an up quark has a mass of about 4-5 MeV (in natural units) and a down has a mass of 7-8 MeV; based on that, you might think a proton has a mass of around 17 MeV and a neutron at around 19 MeV.
Those guesses are reasonable, and yet they're only about 2% of the actual proton and neutron masses, which are about 938 MeV and 940 MeV, respectively. Where does the rest of that mass come from?
From binding energy. Those numbers I gave you for quark masses are for theoretically free quarks, which aren't bound at all to anything else. But -- at least in the Universe's current state -- free quarks can't exist, thanks to the rules of Quantum Chromodynamics, the laws that govern the strong force! Quarks can only stably exist in bound states, and the only bound states of quarks that are stable for longer than a microsecond are protons and neutrons.
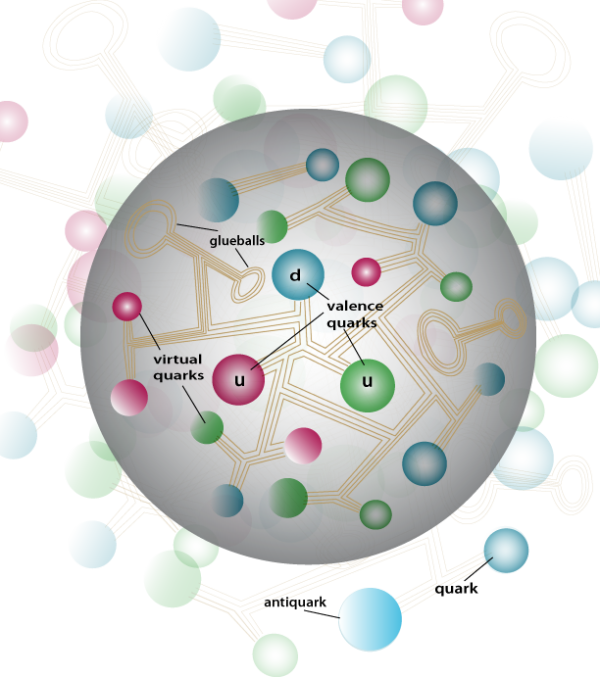
So most of the energy that we observe as "mass" in a proton or neutron doesn't come from the masses of the three quarks that define the nucleon themselves, but rather from the field of the strong force that keeps them bound together. Another way of looking at it is to consider the gluons and the sea of virtual particles (quarks and antiquarks of all types) that make up each nucleon as what really makes the mass of a proton or neutron as heavy as it is.
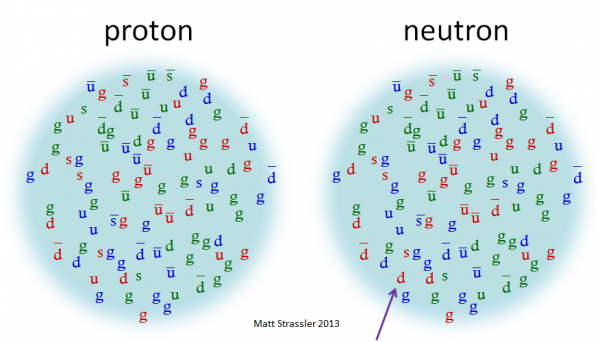 Image credit: Matt Strassler of http://profmattstrassler.com/.
Image credit: Matt Strassler of http://profmattstrassler.com/.
Which is to say, to have something just as simply as one proton or one neutron, there's a lot more involved than just three quarks. In fact those three quarks that you hear of as making up a proton or neutron are more specifically known as valence quarks, and all the other quarks-and-antiquarks inside are known as sea quarks, which carry some 98% of the masses of these particles.
But with the sole exception of a single proton (which serves as a common hydrogen nucleus), these nucleons don't exist in isolation in nature.
 Image credit: CERN / European Organization for Nuclear Research, http://www.physik.uzh.ch/.
Image credit: CERN / European Organization for Nuclear Research, http://www.physik.uzh.ch/.
They exist in states where they're bound to one another. That's what makes atoms interesting: the fact that they have different numbers of protons (which makes for different elements) and different numbers of neutrons (which makes for different isotopes). And unsurprisingly, each unique combination of protons and neutrons has a unique binding energy, and only a very select few combinations are stable.
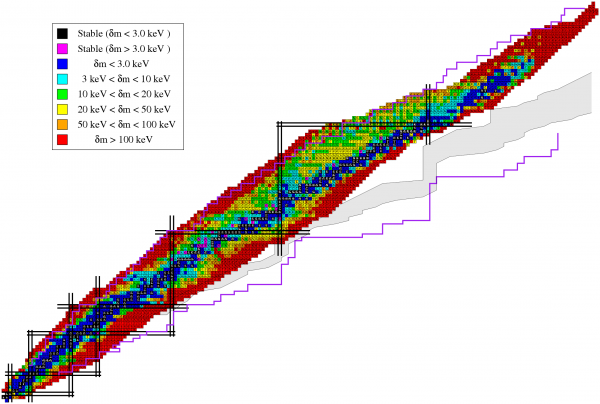 Image credit: I. Cullen at http://personal.ph.surrey.ac.uk/.
Image credit: I. Cullen at http://personal.ph.surrey.ac.uk/.
The simplest combination -- one proton and one neutron -- is known as a deuteron, and is about 2.2 MeV lighter than a free proton and neutron alone. Start adding more, like two protons and two neutrons (to make Helium-4), and you're suddenly 28 MeV lighter than those four free particles. The most stable of all the elements is Iron-56, with 26 protons and 30 neutrons, and which has a mass that's a full 492 MeV lighter than 26 free protons and 30 free neutrons.
Heavier elements and isotopes may have a larger total binding energy, but no element has a higher binding-energy-per-nucleon than this isotope of iron.
Low-mass particles are easy to fuse into higher-mass ones; they emit a lot of energy when they do so. So long as you can achieve sufficient temperatures and densities, this is something that happens spontaneously, and is both the great hope of commercial nuclear fusion and also how all the elements in the Universe heavier than lithium were produced: in the nuclear fusion furnaces of stars!
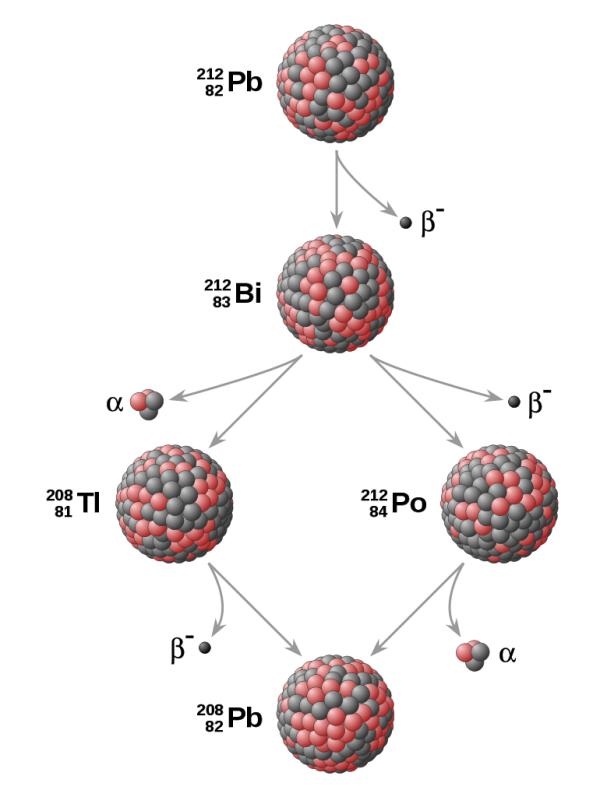
On the other hand, (mostly heavier) particles that have too little binding-energy-per-nucleon can spontaneously undergo one of three radioactive decays to reach a more stable state:
- Gamma decay, where a nucleus emits a gamma-ray (high-energy photon) to form a slightly lower-mass nucleus with the same number of protons and neutrons;
- Beta decay, where a nucleus emits an electron (and an antineutrino) to form a nucleus with a slightly lower mass, with one more proton and one fewer neutron than its parent nucleus;
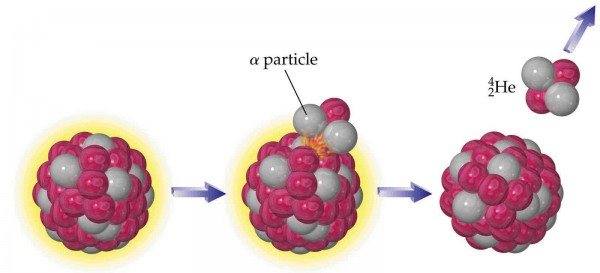
- and Alpha decay, where a nucleus emits a Helium-4 nucleus (two protons and two neutrons, known as an alpha particle), and results in a nucleus with a lower mass, two fewer neutrons and two fewer protons than its parent.
These processes may be a one-off, as in the daughter nuclei they give rise to may be stable, or they may be part of a radioactive decay chain, which can require many steps until something stable is reached.
What's particularly interesting is that:
- Each combination of protons and neutrons has a specific binding energy and therefore a specific rest mass,
- but there are only three types of radioactive decay, each which gives rise to a photon (massless), electron (of a small, given mass) or an alpha particle (of a larger, given mass), and therefore
- each radioactive element produces radioactive decays with specific, characteristic energies (and timescales) associated with them!
Each type of particle -- alpha, beta and gamma -- takes different types of material to stop them, and to shield sensitive things (like you and me) from them.
Alpha particles are mostly harmless; they can be stopped by a sheet of paper, and even if they actually reach your body, are stopped by the outer one-or-two layers of skin cells on your epidermal layers.
Beta particles can do some damage; they can penetrate your skin and, in large doses, can give you radiation sickness and kill you.
But gamma particles are the most deadly: it takes a full foot (30 cm) of lead to effectively shield you from gamma radiation, and most cases of radiation sickness related to, say, the Hiroshima bomb came from gamma radiation.
But that's not all cases of radiation sickness.
Alexander Litvinenko was famously poisoned by being forced to ingest a radioactive isotope of Polonium (Po-210), which is an alpha-emitting radioactive particle. Although alpha decay is harmless if it takes place outside of your body, inside of you, all of that radiation is absorbed internally, and death is inevitable within a matter of days. (This is true for ingesting sufficient quantities of any radioactive material with a short half-life!)
But there are some specific characteristics that allow us to determine just what it was that poisoned him.
When an alpha particle is emitted from a nucleus, it will have a specific amount of kinetic energy that's determined by the decay parent (Po-210 in this case) and the large, daughter nucleus (Pb-206, lead, which is stable). So when you want to determine whether there's a particular radioactive substance, you look for alpha particles with a certain energy, and that's a smoking gun for the radioactive parent.
There's a question burning up around the world right now: is this how Yasser Arafat died?
 Image credit: Arabs48, via http://www.imemc.org/article/64141.
Image credit: Arabs48, via http://www.imemc.org/article/64141.
Although at this point I don't think anyone can say for sure, this is the type of detective work that will uncover the answer: nuclear physics! It's an incredibly powerful thing; used irresponsibly, it can kill hundreds of thousands in an instant or poison a targeted individual slowly and painfully, but used responsibly, it can be a practically infinite source of power for mankind.
It's to be respected and valued, and only feared in the wrong hands. No matter what, it's a great opportunity to understand our natural world a little better, and how matter on the smallest scales can impact the largest things we know!





I've wondered, why do we usually talk of three kinds of radiation, and leave out the neutrons? It's one thing if we don't want to give them another Greek-letter name, but it does seem odd to leave them out of the count.
A few comments from a radiologist/nuclear medicine doc.
When MR imaging came on the scene in the early 80's, it was known, naturally enough, as NMR (nuclear magnetic resonance) imaging, since it derived from the physics used in every chemistry department, the NMR machine. Pretty quickly, the N was dropped purely for PR reasons.
There are a few therapeutic uses for alpha emitters. Primarily iodine -131 treatment for hyperthyroidism and thyroid cancers. The thyroid will take up about 25% of the entire dose of iodine given to a patient, with the rest being distributed throughout the body, and not causing any effect. The alpha particles deposit their ~ 1Mev of energy within a mm or 2 of the site of decay (the thyroid cell) and as a result essentially burn the tissue. This treatment has been around since about the late 40's and is incredibly effective. Also, since the effect is only on the very immediate area, it is incredibly safe. If only we had more like it.
Strontium 89 is used in a similar manner to treat bone metastasis, typically from prostate cancer. It will relieve pain, but not cure the lesions.
There have been other efforts to specifically target pathologic tissue, using, for example, I-131 labelled antibodies, but the antibodies to the tumor do not target the tumors specifically enough to be useful.
There is another decay mode not mentioned in the text, and that is positron decay. Most of the lighter elements, which tend to be the ones we are built on, like C, O, N, etc have an isotope which undergo positron decay. These can be used for imaging, the decay mode produces a 1.022 Mev Positron, which finds an electron within about a mm or so, and produces, via matter-antimatter annihilation, two 511 Kev Photons that exit the body in 180 degree opposed directions. The typical isotope used is f-18, placed on a glucose molecule. This is treated like cold glucose by the body and goes where any glucose would go, mostly to the brain, muscles, and heart. BUT -cancers generally are using a lot of glucose, so this is the basis of PET scanning for cancers. It is fun to tell patients that we are using antimatter to scan them.
There are some other positron emitting isotopes of the physiologic elements, but the half lives are seconds to minutes, making them tough to work with (F-18 is about 2 hours). In the 70's at Sloan Kettering in NY, they installed a pipe under the street from the cyclotron to the nuclear medicine department to immediately deliver 0-15 to the patient (half life 2 minutes), in order to do scans.
@phil - I-131 is primarily a beta emitter. Some alpha emitters are used, like Ra-223 for prostate cancer, but mostly for research purposes.
Hi Ethan
You chose diagrams that illustrate nuclei as tidy arrangements of well defined neutrons and protons, the interior of which seems to be anything but well defined. Do the quarks of individual protons and neutrons remain bound to each other in a special way, or is the whole nucleus a probabilistic Quark Soup like your neutron/proton illustrations? At a quantum mechanical level is this even a meaningful distinction?
Usually, bound states remain bound because their total energy is less than the energy of the free constituents, i.e. the binding energy is negative.
But for quarks inside a proton or neutron, the binding energy is positive! So why do they remain bound? Probably this is due to the fact that free quarks can't exist (confinement).
But how is the binding energy even defined in this case? (It can't be "energy of the bound state minus energy of the state with free particles", because free quarks don't exist.)
Existing as a free quark would require the creation of more energy to create a partner, Bjoern.
It would also have to exit the nucleon and, from the point of view of the binding forces and energy, this is a journey to the moon by shoe leather.
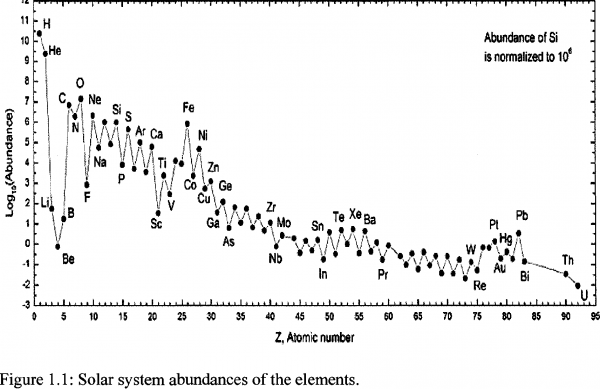
Quick question. For most of the nuclei heavier than boron, it appears that the binding energy per nucleon alternates higher then lower for the next element. For instance, binding energy per nucleon declines going from carbon to nitrogen, rises from nitrogen to oxygen, declines again from oxygen to fluorine, etc. This pattern seems to hold most of the way up to bismuth or so. What is the cause of this?
Helium.
2p2n is a highly bound system. Therefore an even number of p gives you Helium atoms bound in conglomeration and therefore any "spare" protons are not as highly bound as it would be if there were another one to bind with.
@Randy Owens #1: Basically, because neutron radiation doesn't exist "in nature." That is, the radioactive nuclei that you will find by digging up rocks, or by looking at the atmosphere hit by cosmic rays, only decay via beta or alpha emission (with small fractions of channels like electron capture or internal conversion, which produce gammas).
You can get neutron emission in two ways: either you bombard the nucleus with a projectile, such as another nucleus, or gammas; or you start with a nucleus so heavy (heavier than uranium) that it spontaneously fissions. In either case, the result is a couple of smaller nuclei plus one or more neutrons.
I'm confused by the description of gamma radiation. After the emission of a gamma energy photon, the resulting nucleus would have to be lighter, but the description given implies that it is the same element & indeed, isotope (same number of protons & neutrons).
If this is true and the binding energy is the supplier to make the gamma photon, wouldn't this be yet another form of the element beyond the isotope? Should this be an additional number for an element of the periodic table?
Could you pump a nucleus to a higher energy & create a gamma laser?
Obviously, I'm not a physicist, just someone hoping to understand, but what am I missing?
Thanks much.
@Stellar_ash #11: The protons and neutrons in nuclei have "energy levels", just like the electrons in an atom. A nucleus can have a number of "excited states", with the same composition, but with their nucleons in different energy levels.
When such a nucleus "relaxes" or "de-excites" to its ground state, it does so by emitting gamma rays with characteristic energies (just as excited atoms emit visible spectral lines.
Thank you, Doc
Only feared in the wrong hands! How does the science of human nature inform us as to this statement? Now THAT is the quintessential question. Scientists are as prone to human frailties as anyone else, including greed, malice, and psychopathy. Then there's simple mistakes. Scientists have lied for money, and done far worse vis-à-vis cigarette company claims right up to Nazi experiments. Now you say "trust us" again? My answer is a question : how can we?
Ethan
An excellent explanation. I particularly appreciate your clear explanation of proton and neutron mass.
Michael Kelsey
As usual your explanation #9 and #12 are great.
Wow
Thanks for those clear explanations #6 and # 8.
I will have to reread this post and the stream of comments a few more times.
I do have one questions. Taking a look at that Matt Strassler image of the proton and neutron. We see all the quarks and antiquarks; and as well we assume all the various color/anticolor gluons. Now my question is about confinement.
Within a proton or neutron, we certainly have the virtual gluon environment within which glueballs could form. And presumably then those glueballs would be stable enough to be real (rather than virtual particles) and capable of exiting a proton or nucleon. (or NOT, I defer to experts) So can someone give a brief update on the search for glueballs?
I'd just like to know how is the search for glueballs going? And even could glueballs masquerade as something like dark matter? Just asking, maybe even the wrong questions. So, I defer to experts. Thanks.
"I’d just like to know how is the search for glueballs going?"
It's at a sticking point, I think.
Wah wah waaaaaah!
First, to Ethan - what, no love for B+ or EC decay? If you're going to educate the curious, you can't leave out EC! It has the distinguished honor of being the only form of radioactive decay that can, in principle, have its decay rate affected by non-nuclear reactions (take away all the electrons, there is no EC).
Sean @7 - the 30-second answer is that nucleons are more stable when they can pair up. That translates into the even-proton nuclei generally 'holding together' more often when you produce them by slamming two lighter nuclei together. So that saw-blade pattern you see represents slightly higher abundances for the even Z elements, because all their protons are paired up.
While this slightly changes the abundances of the stable elements, it has a far more dramatic effect on the discovery (or production) of new elements. Even wonder why we've discovered 118, 116, 114, but not 117? Its because the even-proton nuclei hold together longer, allowing us to detect them.
Last little tidbit, the same is true for neutrons (nuclei vith even numbers of them tend to be more strongly bound together than nuclei with odd numbers of them).
Michael @9 - yes it does. Uranium and thorium ores emit neutrons 'in nature' as they fission. So does primordial Pu, if you can find any. :)
eric, neutrons contribute to the nuclear force binding over the short range they operate over, and do not contribute to positive electrical potential as protons do in the positively charged nucleus.
Therefore the 2p2n helium nucleus contains the most nuclear binding with the least electric repulsion in the smallest space possible.
Since the electrical force is infinite in range whilst the nuclear force limited, as you get a larger and larger nucleus, the ability of the neutron to add nuclear force binding becomes irrelevant, and only its ability to reduce the electrical density in the neutron remains, hence it becomes slightly better as nuclei get heavier and heavier to put a few neutrons in the mix over and above that needed to create another helium nucleus to spread out the positive charges and reduce the repulsion they feel for each other.
Wow @18 - yes, I know all that. What does it have to do with the fact that nuclei with even numbers of neutrons are generally more stable than 'neighboring' nuclei with odd numbers of neutrons? Your first sentence is basically agreeing whith what I said in my post...all the rest seems to be a different (reasonable and legitimate) point altogether.
" Uranium and thorium ores emit neutrons ‘in nature’"
Only by virtue of moving over to new decay products and finding an embarrassment of riches in the neutron department and wanting to eject the remainder.
The reason why they don't really appear in any definition of radiation is rather like the reason why neutrinos aren't counted in there either: they really don't do much.
The bit the neutron can hit is about 1:10^11 parts of what constitutes even the densest matter we find here on earth.
Therefore they don't really get in on the act.
However, a certain type of uranium when presented with a slow (and hard to find in nature, because nuclear excess energies are generally in the MeV range rather than KeV and less) neutron, that neutron will find it accepted more readily by the nucleus which will then spit out three neutrons that share the excess energy and can be slowed down by enough dense and unreactive material (carbon is a good one: solid at STP and hard to break up, being three helium nuclei hanging about together) to produce neutrons that have a slightly better than 1:1 chance of initiating another reaction with another uranium nucleus of the same type if you've put enough together in a small space.
Which is why you need to refine the stuff a lot to make a useful nuclear reactor.
But the neutrons from most decay products, unlike alpha and beta (and, of course, the photons) do bugger all to anything nearby.
"What does it have to do with the fact that nuclei with even numbers of neutrons are generally more stable than ‘neighboring’ nuclei with odd numbers of neutrons"
It explains it, eric. That's what it has to do with it.
And it explains why it doesn't hold for long in the atomic number.
Your explanation would have 6p0n as a stable atom.
It isn't.
Moreover, the query was about increasing proton, not increasing neutron. Indeed your response was only about the protons, not neutrons.
Quite why you then decided to make it about neutrons instead must be a mistake and you meant protons, hence I treated it as such.
"Mass defect or mass packing fraction" are terms also used to express the difference between bound and unbound particle masses. How can the same thing measured together or separate, give two different masses?
How can the parts of a nucleus, the Proton plus Neutron, be heavier when separated, lighter when together?
Could there be a clue in the standing waves that Tesla detected in his laboratory in 1911?
Standing waves, zero point fields or static fields, all the same thing. They are inertial fields that establish gravitational stability in and of the 4th dimension. Might the binding energy itself not be a factor of universal forces? Using high energy split fields, waste of any sort can be transferred into the 5th dimension, out of our world, into the background of outer space, using the static field.
Whatever the case, It appears that the greatest threat most of us may face is in the form of alpha particles spread. Eating or breathing them in is a problem. Think of breathing a miniature particle sized microwave oven in that keeps running 24/7 from now until eternity. Can we not think of a crop planted in a field, that tries to survive, while the sub atomic micro wave oven is running full time in its roots?
Those close to the overheating reactor, also face gamma and Beta along with Alpha particle issues.
It is understood how to decontaminate alpha particle toxins from our food, but as the ordinary people do not control the issue of our money, nothing is being done to protect us here. Ordinary dust in our air will carry alpha particles into our lungs or rain it on to our food.
How many consider it possible that the Hitachi-GE leaky reactor is a well planned op to contaminate and poison us real well? They refused Russian help from the beginning. Is there some conclusion that we might come to, other than we are in a well staged op to poison us HUGE?
Velocity pumps can decontaminate food and drink, but we don't have them on the shelf as part of our technological world yet. Where is the money for development of static field technology? The issue of money is still held in private hands. What have they done with our purse other than build Nuclear blast and leaky power plants? Must labor not act sensibly and take our purse away from them?
Must labor not STRIKE THEM OUT and get hold of the issue of our paper here? Can we not then hire us some protection from this sparkle waste that is falling out onto our fields and into our air?
Here's a page that shares some theoretical about velocity cures:
http://bitchworld.weebly.com/the-four-elements-of-free-energy.html
@eric #17, Wow #20: Eric, the three natural isotopes of uranium are U-238, U-235, and U-234. The others are short-lived products from either reactors or from alpha absorption on thorium.
You are quite right that neutron radiation occurs in minerals containing U/Th. These come from the interactions both along the decay chain, and from induced reactions (e.g., alpha-n as I mentioned above).
Such neutrons are one of the dominant backgrounds for my own experimental work! I'm a member of the SuperCDMS collaboration, looking for dark matter. Neutrons from the U/Th in the cavern walls, and even worse from U/Th contaminants in the materials of our apparatus, are the main source of false nuclear-recoil signals in our detectors.
@Wow #18, #21, #22: Eric was more correct than you are on this issue. Nuclear stability is driven by Pauli pairing of the protons, and of the neutrons, _independently_. Even-even nuclei have maximal binding energy compared with their neighbors (with 4-He being the extreme example). Even-odd and odd-even nuclei have slightly lower binding energies (and hence tend to decay to an even-even neighbor), while odd-odd nuclei are the "least stable" in this sense.
Your argument for "alpha clusters" in nuclei is an over-simplification. While a useful mnemonic, it is really only useful as such for lighter nuclei (say, nickel and below).
Heavy nuclei always have a neutron excess, because the strong binding from the neutrons is required to overcome the Coulomb repulsion of the protons. Your silly straw man example of a 6-proton cluster is an obvious instance of that, and is often a homework problem in nuclear theory.
No, Mike.
He missed out a hell of a lot.
And this:
"Heavy nuclei always have a neutron excess, because the strong binding from the neutrons is required to overcome the Coulomb repulsion of the protons."
is wrong.
Nuclear force range is...?
Moreover you appear to be including a shitload in eric's post that wasn't there.
Go read it again for what IS there, don't go including things you know "ought" to be there because you already know how nucleons bind in a nucleus.
No, you are confused. Reread my post. I was not telling Sean that p number = n number is inherently more stable, I was discussing the fact that nuclei with even numbers of neutrons (2, 4, 6, 8...) tend to me more tightly bound than nuclei with odd numbers of neutrons (1,3,5,7...). Though odd-odd nuclei can be more stable than either even-odd or odd-even.
What you are talking about - neutron mitigation of charge repulsion - is responsible for the overall slope less than 1 seen in chart of nuclides (the figure with "image credit: I Cullen" under it). But Sean wasn't asking about that chart. He was asking about figure 1.1. And nucleon pair formation is responsible for the alternation that Sean T sees in figure 1.1.
Again, you seem to think I said something I did not say. I was not referring to the ratio of protons to neutrons.
What I actually did say ("nuclei vith [sic] even numbers of them [neutrons] tend to be more strongly bound together than nuclei with odd numbers of them") does hold, in fact, generally across the entire chart of the nuclides.
No, it wasn't a mistake. An aside, yes, but not a mistake. If you look at the chart of nuclides and read across the boxes in a given line (i.e. at the isotopes of a single element),* you will see an alternation in stability. That alternation is caused by neutrons pairing with another nucleon.
*And for goodness' sake, go higher than carbon. You know as well as I that the effect I'm talking about doesn't kick in until about Z = 16.
Michael, here's illustration of why you're wrong on the neutron thing:
Why isn't it Cobalt-54 that is stable?
* No, you are confused.
No I'm not.
* Reread my post.
I did. I read what you wrote, not what you meant when you wrote it.
* I was not telling Sean that p number = n number is inherently more stable
Ah, well, you're confused. Reread my post.
I did not claim you were saying that. Go reread your post after reading mine again and see where you made your mistake.
* I was discussing the fact that nuclei with even numbers of neutrons (2, 4, 6, 8…) tend to me more tightly bound than nuclei with odd numbers of neutrons (1,3,5,7…).
Go re read Sean's post. Here's an excerpt:
"binding energy per nucleon declines going from carbon to nitrogen, rises from nitrogen to oxygen, declines again from oxygen to fluorine, etc."
Notice that the change here is by protons. That's how you change from one element to another.
The reason for this is Helium.
2p2n is helium. Highly bound. 3p3n is helium plus deuterium, 4p4n is two helium.
This goes up for a bit then you need more neutrons and eventually the binding per nucleon is lower because to get a stable isotope you need more than just two nucleons to go up to the next element, but three. Then you need four.
* I was not referring to the ratio of protons to neutrons
I know.
Which is why I said you were incomplete in your explanation and that this incompleteness is what MY response had to do with it.
Did you even read what you asked?
* No, it wasn’t a mistake. An aside, yes, but not a mistake.
OK, then you refuted your own claims about protons because you then segued into neutrons in a question about protons.
You know, that element change thing again. Needs protons. Or it isn't another element, it's an isotope.
And yes, it takes a few protons before the effect kicks in.
The effect, however, kicks in.
Ignoring it doesn't mean it doesn't exist, dear.
@Wow #29: "Why isn’t it Cobalt-54 that is stable?" I am so glad you chose that example, because it exactly illustrates my point (and why you are mistaken about this issue).
54-Co has 27 protons and 27 neutrons. It is an odd-odd nucleus, and what's more, does not have enough neutrons to overcome the Z=27 Coulomb repulsion. Therefore, it is not stable.
The _stable_ isotope is 59-Co, which has 27 protons (duh) and _32_ neutrons. Notice two things; first, the stable nucleus has an _even_ number of neutrons, meaning all filled Pauli pairs, and second, there are five "extra" neutrons compared with the light nuclei Z=N pattern. Those extra neutrons are just enough to push the nuclear binding (i.e., the Yukawa exchange of pions) above the Coulomb repulsion. As Z increases further, the neutron excess has to get larger and larger (for 238-U, for example, Z=92 but N=146).
@Wow #28: You wrote, "
[quoting me: ]“Heavy nuclei always have a neutron excess, because the strong binding from the neutrons is required to overcome the Coulomb repulsion of the protons.” [end quoting me]
is wrong.
Nuclear force range is…?"
1) My statement is not wrong. Please take a look at the chart of nuclides, and note the slope of the Z vs. N axis. You will see that that slope is significantly less than 1, because N > Z for stable nuclei much beyond iron (i.e., _heavy_ nuclei).
2) The binding force between nucleons is nuclei is very well described by an effective Yukawa potential (exp(-l/L)) driven by the exchange of virtual pions (and other light mesons). The characteristic range of this potential (L) is several femtometers, or about the diameter of a litium or beryllium nucleus.
* I am so glad you chose that example, because it exactly illustrates my point (and why you are mistaken about this issue).
And here's your problem: you're thinking that this demonstrates I have a problem.
You would have thought someone would think it through, but hey, no, not you, eh?
Notice how the number of neutrons being 27 aren't enough to make it stable. Therefore your assertion
"Heavy nuclei always have a neutron excess, because the strong binding from the neutrons is required to overcome the Coulomb repulsion of the protons.”
is wrong.
I know what you *mean* but that isn't what you're *saying*.
You see if your assertion there were, as written, correct, then the 1 proton and 1 neutron would bind nicely because *according to the statement made* the "strong binding overcomes the coulomb force.
Two neutrons don't bind to a proton to make a stable Tritium. As an example.
Two neutrons will bind together and do so to exclude a proton coming in, but they do not interact in the coulomb force interaction themselves.
They are filler.
They space out the other constituents that ARE charged (2p2n) and reduce the energy needed to keep all those doubly-charged 2p2n as far away as possible given the strong force would like to make them all meet as closely packed as possible.
The increasing number required as you get bigger and bigger are because the nuclear force is short-ranged, being passed about by a massive particle, whereas the electric force of the positive charges are carried by massless particles, hence are unlimited in range.
Like I say, I know what you *mean*, but you're remembering everything you know but cannot and/or did not fit in to your posts.
And without that, your posts have been incorrect.
"1) My statement is not wrong"
Yes it is. Hopefully you can see why now.
"The characteristic range of this potential (L) is several femtometers, or about the diameter of a litium or beryllium nucleus."
Therefore a neutron that can only "bind" on that range cannot affect a nucleus bigger than that.
Hence your assertion re the neutrons cannot be true except in the trivial case of "when the nucleus is no bigger than that".
Which is rather eric's "point" that the effect doesn't begin to matter until around atomic number 16.
As an example, why does Co54 decay to Chromium?
(note, I assume it would be the next decay product, based solely on the heaviest element that has 54 nucleons, though I had guessed it would be somewhere around atomic number 21, but was off by a couple)
Because neutrons would like to decay to protons.
Therefore the balancing act becomes: does the gain in electric potential if I were a proton instead exceed the energy I'd release if I relaxed to a proton?
Note too: Cr53 is stable. A neutron sitting about all on its lonesome.
Your statements are "true" if you know what you're missing out here, Michael. You do. But someone reading this won't.
But they're only true for some elements and your statement is only *true* true knowing how to work out where your assertion holds and where it doesn't.
And absent that information, it makes your *actual statements* false, because they only contain the context of the information supplied.
My statements are more true within the context of the information supplied, even though it doesn't make a claim on any specific occurrence, just explains the factors. Where it doesn't apply, it's *truly* false.
And unreliable, especially where its not necessary, is worse than wrong when it comes to teaching or informing people. Your statement re: double neutrons (remember, Sean said *nucleons*, not neutrinos) is wrong with Cr53.
Your assertion is wrong because it's a "there are no black swan" assertion.
I made no assertions of the precise colour, just that colours exist. To stretch the metaphor.
Concerning Arafat ,there is no question that he was poisoned by enemies with Polonium and Israel had them available.
Wow,
Michael addressed or is addressing most of your points better than I can, so I'm going to keep this short.
It doesn't. It ECs to Fe54, which has an even number of both protons and neutrons. So this is now the second instance of you providing quite a nice example of what Michael and I are talking about.
Yeah, it's a shame Co54 doesn't decay into Cr53, or you might have a valid point.
In fact Chromium is yet another good example of what we're talking about . The stable isotopes are 50 (even even), 52, (even even), 53, (even odd), and 54 (even even). Notably, 51 is not stable despite the fact that both the immediately lighter and immediately heavier isotopes around it are. This is exactly what I was talking about in my first post, when i mentioned neutron pairing. Cr50 vs. Cr51 is a classic example of how neutron pairing can be more important than the simple charge mitigation effect you're talking about. If it was all about reducing the impact of proton charge on proton charge, then 51 should be more stable than 50. But it isn't - its less stable. Why? Because in 50 all the neutrons pair up, while in 51, they can't.
@Wow various: Let's start with some very simple statements.
1) Forces add linearly.
2) The nuclear potential is the sum of the inter-nucleon potentials (where the sum is really an integral taking account of the distances involved).
Statement (1) means that a comment like "the nuclear binding exceeds the Coulomb repulsion)" is a statement about relative magnitudes, which add to determine the net force (potential) involved.
Statement (2) means that the few-fm range of the individual nucleon-nucleon forces still adds up to determine the net nuclear potential. That integral inlcludes both the exp(-l/L) attractive Yukawa coupling, *and* the 1/l^2 replusion between protons (obviously n-n and n-p pairs don't have a Coulomb term).
I leave it as a homework exercise to perform the necessary sum over all nucleon-nucleon pairs, and the integral over distances, to derive the net nuclear potential.
Once you've done that, please come back and explain, in detail, where my statements were incorrect. If you can't do so, please review the appropriate literature, including first-year graduate textbooks on nuclear physics, to find the well-known form for the averaged nuclear potential as a function of radius.
One last goof of Wow's that I want to address.
In fact, the decay of Co54 involves a proton 'capturing' an s-electron and converting into a neutron.
So not only did you get the product element wrong, you got the nuclear reaction that's occurring backwards.
"Michael addressed or is addressing most of your points better than I can, so I’m going to keep this short. "
He;'s responding to them, but only some of them.
For example, he's continually ignoring as you were what Sean was asking.
Nucleons are not neutrons.
" As an example, why does Co54 decay to Chromium?
It doesn’t."
And here yet again is why you're an ass, along with Mike here.
Did you spot this bit before you gleefully jumped to go "HE'S WRONG, EVERYBODY! LOOK HE'S WRONG HERE!!!!"
(note, I assume it would be the next decay product, based solely on the heaviest element that has 54 nucleons, though I had guessed it would be somewhere around atomic number 21, but was off by a couple)
?
No, you did not.
Just like you STILL don't understand that it was nucleons said, not neutrons. And the example given was a change of proton, not neutrons.
Why?
Because you've staked your self perception, like Mike has, on not being wrong in any factor, form or title.
So you, like Mike, refuse to actually read things if that doesn't generate your needed story.
"@Wow various: Let’s start with some very simple statements.
1) Forces add linearly."
Lets start with point #1 being wrong.
Shit, this is like the prof at university who, seeing two undergrads whistling to see who can get the sharpest peak in the FFT oscilloscope then disdainfully said "Do you think you can get your larynx out to measure it to see how to calculate that frequency?" then regally stalked away.
So lets start with point 1.
It's wrong.
For quite a few reasons, but the most pertinent one is that one force here is infinite in range and the other one limited, therefore the forces do not add because one doesn't get to the entire nucleus.
@Wow #42: In your example, the professor was quite wrong: the undergrads can do the experiment, and use the result to _calculate_ the characteristic dimensions of their vocal apparatus.
In your case you are missing some basic physics. Forces add linearly. Period. If you have a force where the potential has a limited range (like the Yukawa potential V = exp(-r/R)), that just means that in the sum outside that region it contributes zero. The equations are still linear. Period.
For the case of a nuclear potential, the form is a sum of powers of 1/r. For large r, it's primarily a -1/r repulsion (which gives the expected 1/r^2 force). Close to, and inside the nucleus, it's an attractive potential which goes, I think, something like 1/r^4, and then there is a repulsive core like 1/r^7. When you add them all up, the potential has a minimum at some characteristic radius.
As I did before, I encourage you to look up the fine details in a either an upper division undergraduate, or first-year graduate, textbook. You might even choose to read the Wikipedia article (https://en.wikipedia.org/wiki/Nuclear_potential), which repeats what I've written in previous posts, but with better numbers. Or, you can continue to assert your own infallibility.
@Wow #41: Unfortunately, you're the one who fails to understand what Sean #7 was asking. He used the exact phrase "binding energy per nucleon" twice in his question, because that is the exact phrase used to label the Y axis on the plot in question. Perhaps you're so caught up in your own infallibility that you couldn't see the forest for your trees.
He was asking about the alternation in AVERAGE BINDING ENERGY PER NUCLEON as a function of ATOMIC NUMBER (Z), that is, the number of protons.
That _average_ is computed for all of the isotopes of a given element, and so washes out the detailed dependence on N (which also has the same even-odd cycling), which is more visible in the two-dimensional chart of nuclides.
Having averaged away the neutron dependence, the remaining even-odd dependence on Z is exactly what Eric and I explained, that nucleons (being fermions, just like electrons) form pairs with lower total energy than two separated nucleons.
* He used the exact phrase “binding energy per nucleon” twice in his question, because that is the exact phrase used to label the Y axis on the plot in question.
No, I know that.
Nucleon != Neutron.
* He was asking about the alternation in AVERAGE BINDING ENERGY PER NUCLEON as a function of ATOMIC NUMBER (Z), that is, the number of protons.
I know.
Proton != Neutron.
* That _average_ is computed for all of the isotopes of a given element
I know.
* Having averaged away the neutron dependence
Why then keep banging on about neutrons then?
* the remaining even-odd dependence on Z is exactly what Eric and I explained
No, because, as you said earlier in that post:
"ATOMIC NUMBER (Z), that is, the number of protons. "
Yet you kept banging on about neutrons.
"@Wow #42: In your example, the professor was quite wrong: the undergrads can do the experiment, and use the result to _calculate_ the characteristic dimensions of their vocal apparatus."
He was a shitload more wrong than that, kid.
You don' t use your vocal chords to whistle with.
* In your case you are missing some basic physics. Forces add linearly. Period.
You're wrong. PERIOD.
A force that doesn't go that far doesn't add at all.
You are adding zero.
The force doesn't go there, so it adds zero. Which means it isn't adding at all.
@Wow: I don't need the abuse you enjoy heaping out to the other readers of this blog. You don't listen, you can't read, and you don't seem to understand the basic physics you so abusively deride others for.
I have tried to correct your misunderstanding clearly and directly, while still being polite. You have not. Any further abusive language on your part will lead to a request that you be banned from these fora.
Mike, that abuse is a figment of your wounded pride, dear.
Did I call your fatuous bollocks about how I don't understand something merely because you'd like to talk down to me as if I'm some pre-schooler, abuse?
Fuck no.
Why?
Because I'm actually an adult.
Not a moronic little toerag who isn't listening to a damn thing in case they hear something that doesn't get their ego stroked.
I've tried to show you where you're wrong, but you've 100% ignored it and instead given baby talk in a pretense to belittle me.
Then you have the unmitigated gall to whine about "abuse" and fellate yourself on how "polite" you've been because you're not used words you've decided are not polite.
Polite isn't just not using "fuck off", you braindead moron.
It's actually listening to someone when they say something.
Wow
Here's the thing Wow.
You are abusive. And it gets very tiring.
The thing is, I can't imagine that in person you talk with people the way you do out here on this blog. Because in person, someone a lot less polite than Michael Kelsey would punch your teeth in.
So I assume that out here you take on a different persona.
Whereas out here, I assume that Michael Kelsey, or Ethan have the same persona as they do in daily life.
So I would suggest that out here on this blog; that you try to give people the same kind of attitude that you do in your day to day life.
Now if you say that you carry the same attitude in your daily life with the grocer, the taxi driver, the street preacher and so; then tell me, Do you still have all of your teeth?
Personally, Wow, I am not for banning you from this blog.
1) I think you do understand and contribute to the science discssion.
2) You do fight the antiscienc folks tit for tat
3) Your venom and rudeness has diminished over the years (We all grow).
Psychologically, having an integrated personality, is not the norm. It is rather unusual. The various masks and attitudes that people have and carry change like the weather; and it is the rare person with the personal integrity to objectively observe and be self critical of their various unintegrated persona, masks and attitudes.
Of course some scientific folks think that psychology is nonsense and not science. They are wrong. The first requirement of a scientist is to be objective and this is not possible if they are not able to look themselves in the eye and acknowledge their biases and limitations; personal as well as professional.
You see, my guess is that in your personal life, that you Wow are a bit of a mouse. And you need to better integrate online persona and your day-to-day life persona; to the benefit of both. Yes, bring more appropriate anger to your day to day life; and you will be surprised that much of your inappropriate blog anger will fade.
Wow
Try appropriately expressing some of your online blog anger in your offline day to day life.
I suspect that you are a bit more civil in your day to day life (or else I suspect you are missing a few teeth).
Now if you can learn to appropriately express your anger in the flesh; then I predict that online on this blog, you will find that your need to be inappropriately angry and rude will disappear.
Yes, yes; this advice is coming from someone who has spent a lot of years in psychotherapy. I told you once before Wow, during an online argument that I do not know anyone who is more angry than I. Mostly, it comes out appropriately now. It's taken a lot of years of emotional work.
Wow, I do not think that you should not be banned from this blog. What I do believe is that if you were in a coffee shop talking to Michael Kelsey; that you would be quite civil and not a bit rude. And I suggest that you can learn to bring your day to day civil behavior to this blog. It is possible to disagree and stand correct and yet be assertive. there is really very little reason to be rude. Being rude is evidence that we are helpless and insecure and need to work on that. I also suspect that in coffee shop that you would be inappropriately silent and you need to be more assertive in the flesh.
Yes, yes, Wow, I expect you to pummel me for these remarks.
Fine but think upon what I say.
Don't delude yourself and pretend that there is no truth in what I or Michael Kelsey say.
F me, I started reading this thread thinking 'Wow has really calmed his ass down over the last few months, can be really quite helpful nowadays' and BOOM. Mr Hyde returns.
Seriously dude - read all that back and imagine you didn't write it. See how it looks.
"Because I’m actually an adult... Not a moronic little toerag... Polite isn’t just not using “fuck off”, you braindead moron."
Can you for one second picture that exchange in a face-to-face discussion not ending in blows?
This isn't the comments for an Alex Jones Youtube video. Nor is it a pissing contest where 'one side must win'. You can always agree to disagree, too. Or disagree on semantics, if not the physics.
I've no idea which of you is correct and I don't care. Hiding behind anonymity is allowing you to stoop to levels you would never do in real life (I hope).
Take a breath, calm down. Just no need for that.
Fuck off, mcandrew.
Instead of looking to see how I'm wrong, how about just fucking reading the stuff, eh?
eric gives an explanation that only holds for a short while and I explain to eric why it's not right to say so, he then goes "What does that have to do with it?" Well, what it has to do with it is "It's telling you where you're wrong".
However, this is ignored as it doesn't gel with the self image eric has of either himself or me.
So he then starts wibbling on about neutrons when the query was about nucleons and the EVEN COUNT of protons.
So I point it out and then eric and Michael jump in with a whole load of wrong shit, ignoring EVERYTHING said in a base attempt to shout down someone who uses ruder words than they like people to use.
Then YOU come along and go all concern trolling and waving your flaccid e-peen all over the place to show how brilliant you are.
Well fuck off.
Read the shit before you jump to a conclusion, moron.
"Try appropriately expressing some of your online blog anger in your offline day to day life."
I do.
Try not pushing a derogatory and demeaning attitude and life onto other people you know FUCK ALL about.
Trying that shit in real life will lead you to be wearing your arsehole around your shoulders, your arse will be that kicked.
"I suspect that you are a bit more civil in your day to day life (or else I suspect you are missing a few teeth)."
Based on your presupposition that you only entered into because you want to find fault.
No, I do this IRL too.
Do you know why I get away with it?
Not because I'm 6'8 and 280lb.
No.
Because I'm as hard on my as anyone else.
@wow#46 A force that doesn’t go that far doesn’t add at all.
You are adding zero.
The force doesn’t go there, so it adds zero. Which means it isn’t adding at all.
You seem to be resorting to semantics to win your argument. The term certainly does not contribute to the result, but to a mathematician it is still "added" even if it evaluates to zero.
But my understanding of the above discussion is that it is never zero anyway, it is merely asymptotic to zero. So the forces are additive whatever the distance, and you seem to be contesting Michael's statement of fact by extrapolating from your approximation.
"You seem to be resorting to semantics"
Indeed.
Just like Michael and not, unfortunately, eric, OKThen, MacAndrews.
But somehow you only noted one. Odd how that goes, eh?
If the force carrier goes no further than a femtometer, then something two femptometers DOES NOT ADD TO THE FORCES.
Michael is only correct in so far as that addition is zero.
But apparently pointing it out is "merely semantics" FOR THE WRONG PERSON.
"But my understanding of the above discussion is that it is never zero anyway, it is merely asymptotic to zero."
No, the force carriers are massive. They have rest mass. Therefore their ability to survive has a limited lifespan: even if they carry no energy to impart in the force exchange, they cannot last longer than the uncertainty principle allows. The faster they go, the more energy they can impart but the shorter they can last.
"So the forces are additive whatever the distance"
You DO NOT ADD a force that cannot act.
"they cannot last longer than the uncertainty principle allows"
Whatever others have posted, you seem to be the one arguing from the assertion that "zero" and "too small to matter" are exact equivalents.
It's 35 years since I did my physics degree, so things might have changed since then. In those days the uncertainty principle applied to the result of the product of two variables. Hence if one variable is zero the other must be infinite. You state:
"If the force carrier goes no further than a femtometer, then something two femptometers DOES NOT ADD TO THE FORCES."
Clearly as a conditional statement it is undeniably logically correct., whereas the statement below must become unconditional as our definition of "small" tends to zero.:
Since the force carrier is very unlikely to go more than a femtometer, then the added effect on something at two femptometers is so small it can be ignored.
Can you agree with that statement?
* Whatever others have posted, you seem to be the one arguing from the assertion that “zero” and “too small to matter” are exact equivalents.
They aren't here.
If you do work for me and I pay you nothing, then your payment is zero.
If you don't do anything for me to pay, then your payment is zero.
The two are not equivalent things, though.
Are they.
* In those days the uncertainty principle applied to the result of the product of two variables. Hence if one variable is zero the other must be infinite.
Indeed it is.
However, despite knowing this, you're not seemingly capable of applying it other than in the abstract.
You see the thing is that the force carrier for the weak and strong nuclear forces are not zero mass.
Therefore there's not a zero.
Therefore there's not an infinity.
YOUR assertion only, and I repeat ONLY applies to force carriers that have zero rest mass.
Well Wow, we seem to agree on everything except the final conclusion, so I am clearly missing something. Please can you explain what the exact relationship between magnitude and distance for the force we are discussing is, particularly at exactly what distance does it reach zero?
Mass-energy of a particle: m0.c^2.
Uncertainty principle: E.t<= hbar
Ergo:
t<=hbar/m0.c^2
Therefore if m0 (rest mass) not zero, t cannot be bigger than a set value, and in that case, it cannot have any kinetic energy. If it has no kinetic energy, then the maximum distance the particle will travel is zero.
s=ut
and when u=0, s=0.
If the particle has as much kinetic energy as it has rest mass, it can only last half as long. But at least then it goes a distance.
But the higher m0, the shorter time it can last before it has to poof out or violate conservation of energy.
The distance at which it can no longer exist reduces the heavier the rest mass of the particle involved in the intermediation is.
To the limit of infinity if m0 is zero.
Try here:
http://www.phy.duke.edu/~kolena/modern/forces.html
At least they can use proper symbols.
Here's Sean's observation:
--- it appears that the binding energy per nucleon alternates higher then lower for the next element. ---
Changing neutrons do not change the elements.
Well I did learn something from that exercise, so it was not a complete waste of time. Can I distil the essence of what you are saying to:
"The maximum range of a force is inversely proportional to the rest mass of the associated force carrier"?
But this does not invalidate the statement that "forces add linearly", which you took strong exception too. The equation for total force felt by a particle is the sum of the individual forces acting on it. Not only is this still true when one of the terms evaluates to zero, but surely to a scientist it is the experimentally observed or theoretically deduced value of that term that sets an upper limit on the value of the rest mass of the force carrier.
“The maximum range of a force is inversely proportional to the rest mass of the associated force carrier”
Aye, that's about right.
"But this does not invalidate the statement that “forces add linearly”"
It does.
For the same reason as "You haven't paid me a penny!" changes depending on whether you did anything that needed paying from me.
Hell, I even spent very many words elucidating that although it was "right" technically, since a force that doesn't exist adds zero to the result, BUT YOU DO NOT DO THE SUM OF ADDING ZERO.
And it's that capitalised bit that is really quite important.
The addition of forces that make a photon fly through the vacuum doesn't include the electrical potential force of the charged atom near the sun.
NOT because the force is "practically zero" but because it doesn't interact via that force.
The forces making you move around DO NOT include a zero force component from my pushing you BECAUSE I AM NOT PUSHING YOU.
PS glad to hear you learnt something.
When I was introduced to virtual particles at Uni, the concordance there how the *explanation* for the range of the forces fall out from the uncertainty principle that allows for virtual particles was particularly harmonious to me.
@52:
The pairing effect holds all the time. For both neutrons and protons. And it holds across most of the chart of nuclides; I eyeball it as occurring between Z=18 and Z=108. On the lower end, its overwhelmed by coulomb effects like the one you mentioned. On the higher end, we are looking at single or few-atom detections; such a pattern may simply be lost.
Now I'll try once again, although I'm basically just repeating what I wrote the first time: the effect Sean asked about is due to proton pairing. As a last little tidbit for Sean and other laymen, they might like to know that neutrons pair in a similar manner. This neutron pairing is not directly responsible for the effect seen in Fig 1.1, but it is responsible for the pattern of alternating less-and-more stable isotopes which is seen for most elements throughout the chart of nuclides (like the Cullen figure), from approximately Z=18 on up. It is responsible (to use your choice of example, Chromium) for the fact that 50Cr and 52Cr are both stable but 51Cr is not. It is also responsible for the fact that 48Cr has a half-life thirty times longer than 49Cr.
""eric gives an explanation that only holds for a short while "
The pairing effect holds all the time"
Well given even you said:
"*And for goodness’ sake, go higher than carbon. You know as well as I that the effect I’m talking about doesn’t kick in until about Z = 16."
I wonder why you claim differently.
"Now I’ll try once again, although I’m basically just repeating what I wrote the first time: the effect Sean asked about is due to proton pairing."
No, you nitwit.
Pairing off isn't going to cut it. If that were all of it, then 6p would be stable.
It isn't.
The effect is that 2p2n is very very stable and every even proton number can form one or more Helium nuclei, each of which are very highly bound, therefore the binding per nucleon is a local maxima.
The heavier nuclei require more neutrons to thin out the protons because of the electrical force they suffer under that neutrons don't, whilst both are affected by nuclear binding.
And I draw your myopic view again to this statement:
"proton pairing."
Flip flop flip flop flip flop.
You spent a lot of time squarking about neutrons.
Now you're back on protons again.
Do you have any idea what you're saying, or is it just being made up as you go along, hence all these logical errors you blurt out?
Yes, it holds all the time. Are you now measuring time in amu? Charge units? In some nuclei, other forces overwhelm the effect, but it is still there. Thus your 6p example is just silly. I've never said its the only force; I'm saying its a force and that it explains the sawblade pattern seen in figure 1.1.
The reason your "contains 2p2n" reasoning is wrong is that many of the higher Z nuclei in figure 1.1 do not contain the same numer of protons and neutrons. Thus, they are not solely composed of 2p2n units. But they still show the pattern. Why? Because 2p2n is a derivative effect of a more general rule, and that's the pairing rule. YOUR explanation is good for a few of the lower Z elements but explains nothing else; the pairing explains all of them
I think here you are transparently misrepresenting me. I've very clearly been making two points the entire time, not flip flopping.
I invite anyone to read my second paragraph in @65 and draw your own conclusions about whether I'm flip flopping, as Wow says, or making two points - one about protons, another about neutrons.
Because I think you're now being deceptive, this will be my last post on this thread. Feel free to get in the last word.
eric, if the follow up question were "So why is Helium so tightly bound, then?" your response "pairing" would be in there.
However, "pairing" in answer to the query Sean had is about as accurate and useful as answering "Nuclear forces".
I.e. so pointless an answer it's wrong.
But no, you've heard "pairing" somewhere and you're damn well going to bleat it again and again because you've heard it and it sounds like an answer to you.
And eric, you've not been reading your own posts if you're going to edit them to mean what you've put there.
You may be writing out what you *meant* to say, but that's only what you put in your head. Not what you put into type.
> [me]The pairing effect holds all the time”
>
> [Wow]Well given even you said:
>
> “*And for goodness’ sake, go higher than carbon. You know as well as I that the effect I’m talking about doesn’t kick in until about Z = 16.”
> Yes, it holds all the time.
Ah, I see.
"What's the time?"
"Elephant!"
Which may be the answer to a different question, but it's not the question put or answered.
No you didn't.
You read what you thought should be there.
The binding energy of the deuteron is simple to calculate. Just apply electric and magnetic Coulomb forces statically, without the assumption of nucleons orbiting around nothing.
All details in my paper
http://www.aemjournal.org/index.php/AEM/article/view/218/