"People demand freedom of speech as a compensation for the freedom of thought which they seldom use." -Soren Kierkegaard
After all is said-and-done this week, and after all the new posts over at the main Starts With A Bang on Medium, you've had a chance to have your say here on our forum! And Kierkegaard would likely change his tune if everyone he came across left comments like yours.
From the end of the Sun's life to the light elements, let's take a look at your best comments this week!
From Ted Lawry concerning Ask Ethan #27: Will the Earth and Moon survive? -- "What about drag? The earth would be plowing through all that mass the sun is losing as solar wind?"
This is a reasonable thought; as the Sun expands and gently blows off its outer layers, won't the Earth be plowing into that matter, the way a fast-moving car plows through rain?
In theory, there are two things we'll need to compare:
- The speed of the Earth as it moves in its orbit around the Sun.
- The speed of the matter being blown off from the Sun as it crosses Earth's orbit.
As it turns out, for the vast majority of the matter, it's not even close. On average, the Earth takes about 58 days to traverse the equivalent of the Earth-Sun distance, and on average, particles ejected from the Sun take about 3 days to reach the Earth. In other words, the Solar Wind travels more than ten times as fast as the Earth orbiting the Sun.
And so although the drag force exists, it's very small, and will likely help keep the Earth's orbit relatively circular as it spirals outwards, but won't play a significant role in causing the Earth's orbit to decay and inspiral. It's an important thing to consider, but quantitatively it won't be enough to cause the Sun to devour us.
From Robert H. Olley on Ask Ethan #27: Will the Earth and Moon survive? -- "You seem to be saying that red giant formation coincides with the onset of helium burning to carbon.
According to Jim Kaler of UIUC (one of America’s astronomy heavyweights) there’s a double process, first with hydrogen burning on a helium core and blowing up to an “ordinary” red giant. Then the helium core ignites, and the star contracts somewhat. This is followed by helium burning on a carbon-oxygen core, and the star becomes a bigger red giant.
See the following: http://stars.astro.illinois.edu/sow/star_intro.html#giantsfrom the short section “Giant stars” to “Bigger red giants and Miras”."
Stellar evolution has many stages, and I do my best to summarize what the important points are clearly and succinctly, and so does Jim Kaler, who's excellent at what he does and whom I respect tremendously. The full details of the stages our Sun will go through are summarized in the diagram below, and I'll walk you through it and try to clear things up.
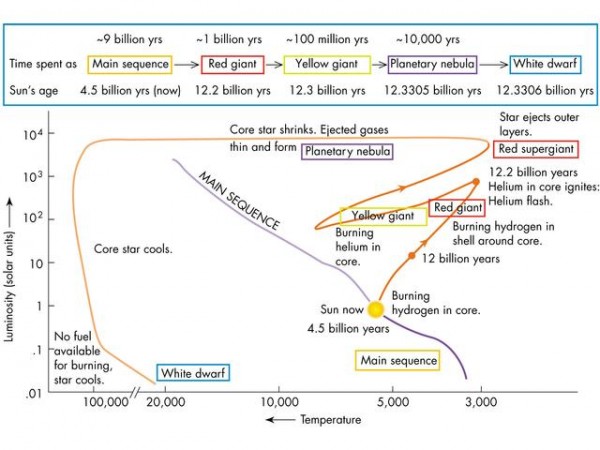 Image credit: SDSS / SkyServer, via http://skyserver.sdss.org/dr1/en/astro/stars/stars.asp.
Image credit: SDSS / SkyServer, via http://skyserver.sdss.org/dr1/en/astro/stars/stars.asp.
When the Sun runs out of hydrogen it its core, it expands into a subgiant and starts burning hydrogen in a shell around the core. It continues to expand and expand as its surface temperature cools, a process taking many hundreds of millions of years, eventually crossing the threshold to becoming a true giant star, and finally the helium in its core ignites. (That's the "helium flash.")
The star remains a giant star for some time, changing colors to yellow and then back to red as the innermost core runs out of helium fuel but helium burning continues in a shell, blowing off its outermost layers most rapidly during this time. (Although, to be fair, it's blowing off its outermost layers continuously during this entire process.) Stellar evolution is a huge, nuanced process that comprises an entire sub-field of astronomy and astrophysics research, and I think Jim does an excellent job, but I don't think anything I said contradicted that. At least, I hope not!
From PJ concerning Messier Monday: A Spiral Sliver headed our way: M98 -- "when you say M98 is headed toward us, do you mean that literally, or are we (our galaxy), in fact, overtaking M98 because its velocity is less than our Milky Way?"
Messier 98 is one of more than a thousand galaxies in the Virgo cluster, a dense galactic group located some 50-60,000 light years away. In the image above, it's visible on the right, with its relatively nearby neighbor, M99, on the left.
 Image credit: Gerard van den Braak of http://www.sterrenwacht.eu/M98-messier.htm.
Image credit: Gerard van den Braak of http://www.sterrenwacht.eu/M98-messier.htm.
Gravitationally bound objects have what we call an average velocity, where we can compute how quickly the cluster is moving relative to us. But each of them also has a peculiar velocity, where they can be moving either towards us or away from us on top of the average velocity. For the Virgo cluster, if we measure the recession speeds of each of the galaxies, they show up in the ellipse highlighted below.
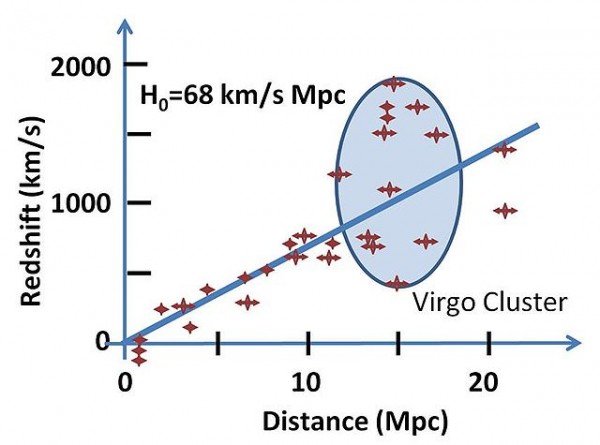 Image credit: forum member Thriveth from http://physics.stackexchange.com/questions/96679/expanding-universe-and….
Image credit: forum member Thriveth from http://physics.stackexchange.com/questions/96679/expanding-universe-and….
On average, the Virgo Cluster is receding from us at right around 1,000 km/s, but galaxies within it can have peculiar velocities of up to 1,500 km/s, meaning that a few galaxies are (temporarily) moving towards us at up to a few hundred km/s (like M98), while some galaxies are moving away from us at over 2,000 km/s (like M99)! They're all going to expand away from us as the Universe continues to age, although for the next few tens of millions of years, M98 (and M86, and a few other large Virgo galaxies) will continue to move towards us before turning around and plunging back towards the center-of-mass of the cluster.
It's literally heading towards us (and getting closer to us), but that's only temporary!
A lovely sentiment from John D. Whitehead after reading Why The World Needs Cosmos -- "I will be 50 this year, and the original Cosmos inspired me as a teen. My grandfather said “Read something and learn every day and you’ll be smarter than any ‘Professor’ and never be in need of a degree or ‘credentials’.” I was about to turn 8 when Apollo 17 ended all hopes of returning to the Moon in my lifetime, and I was short-sighted when it came to realizing that a life of exploration was still open to me. Thank you so much Ethan for rewarding all of us millions who still dream and are always searching the Universe with our questions every day. Your site is the first I go to every night at work, and you answer in a more satisfying depth than the usual spoon-fed pablum the ‘target audiences’ get on Nova and documentaries like ‘Cosmos’. I search out and explore every link, for the deeper nourishment, I have come to crave from a lifetime of learning. Thank you and Thanks to Carl and now Neil for pointing the way to the Cosmos in and around us, you have all made the Journey less lonely for me."
There were no comments on this post that really asked a question or warranted a response, but I felt I should say something about this one. The Universe is something that brings us all together, a story that we all share with one another and with everything else, living and inanimate, past, present and future, from the smallest atom to the largest black hole.
 Image credit: Scientific American, 2004. © 2005 by Don Dixon, original at http://cosmographica.com/cosmo20130812/album/Cosmographica%20Gallery%20….
Image credit: Scientific American, 2004. © 2005 by Don Dixon, original at http://cosmographica.com/cosmo20130812/album/Cosmographica%20Gallery%20….
And our local group will stay with us for trillions upon trillions of years, while the rest of the matter in the Universe will disappear from our cosmic horizon. That's the truth of our reality. To quote Carl Sagan,
For small creatures such as we the vastness is bearable only through love.
Thanks for sharing in the journey we're all taking, and thanks for having me as a part of it.
From Sinisa Lazarek about The 3 Most Surprising Elements -- "I’ve read a while ago that Lithium abundance is significantly off in comparison with theory and observation.
Does this mechanism of spallation correct that or is Lithium data still a big problem. From the article it seems all is ok and fits the theory perfectly. Just wondering if Lithium problem was resolved in last couple of years?"
Sinisa is referring to these measurements, which are close for Lithium-7 to matching what the values ought to be from observations of the CMB, but which miss by a little bit. The predicted values are just a little high compared to what we observe.
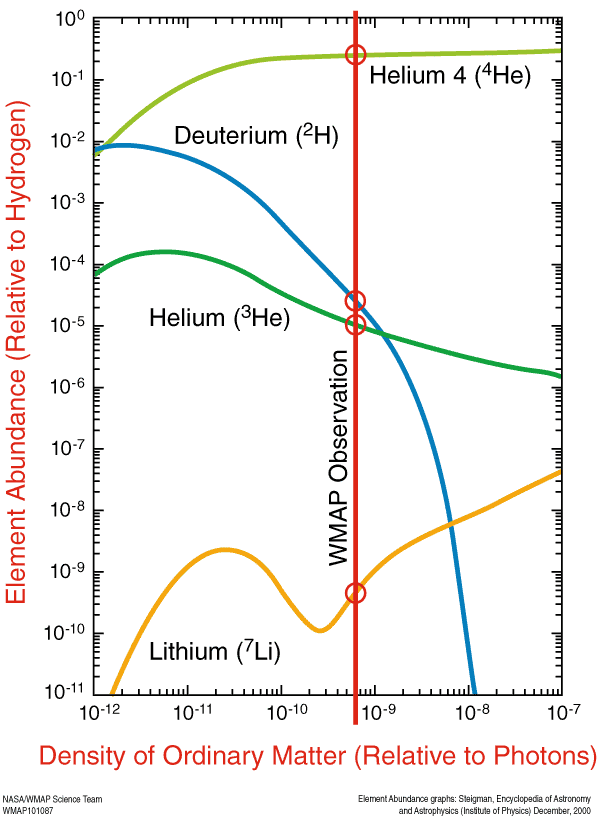 Image credit: NASA / WMAP science team, via http://wmap.gsfc.nasa.gov/universe/bb_tests_ele.html.
Image credit: NASA / WMAP science team, via http://wmap.gsfc.nasa.gov/universe/bb_tests_ele.html.
There's also the Lithium-6 puzzle, where there's a little too much Lithium-6. The problem with these observations is that we are uncertain as to how much of the lithium we see is left over from the Big Bang, how much has been destroyed in stars, and how much has been produce from spallation: from cosmic rays causing the nuclear fission of heavier elements.
It's fair to say that this is an ongoing area of active research; one person I follow quite closely and whom I respect is Karsten Jedamzik. The standard picture of Big Bang Nucleosynthesis is certainly not in jeopardy at this time, but it's worth keeping an eye on.
And finally, from Arun Kumar, also concerning The 3 Most Surprising Elements -- "Loved the article, but I do have a question. How does this explain the concentrations of Lithium that are mined, for the manufacture of batteries for example?"
You see, this is a good question! In the Solar System -- and in the Universe in general -- Lithium is somewhat rare.
But in the Earth's crust, there's actually a significant amount of Lithium, much more than you'd expect from the above graph!
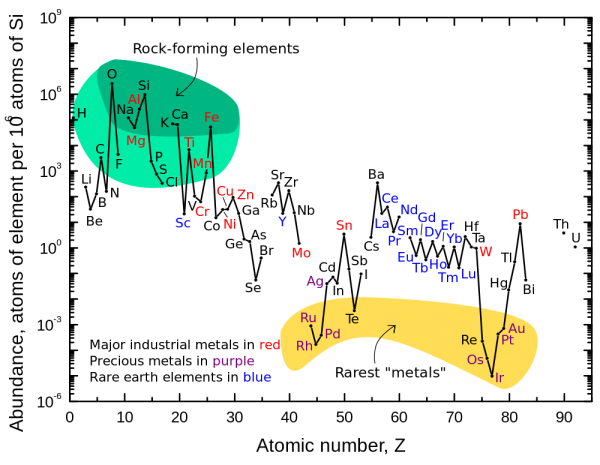 Image credit: Gordon B. Haxel, Sara Boore, and Susan Mayfield from USGS; vectorized by Wikimedia Commons user michbich.
Image credit: Gordon B. Haxel, Sara Boore, and Susan Mayfield from USGS; vectorized by Wikimedia Commons user michbich.
Why is lithium so much more common in our crust? Quite simply: because lighter elements buoyantly "float" atop the heavier ones! While the heaviest metals are concentrated in our planet's core, the lightest ones have preferentially risen to the crust. As it stands, most of the lithium in our planet is located within the first few hundred km of the surface, which is crazy considering our planet is over 6,000 km to the core!
The segregation isn't perfect, but it's good enough to explain why there's a somewhat large amount of lithium in our crust, and why it's not rare to us at all!
And those are your comments of the week!



Am I missing something here? Last time I did any classical mechanics, forces exerted as a result of orthogonal flows are pretty much independent. In other words, no matter how fast the solar wind is moving, the orbital drag is a function of orbital velocity and the density of the matter swept up by the Earth as it goes through the neighborhood.
Otherwise, I don't see how you'd conserve angular momentum.
[1] Oh, for mathml in the comments!
DC: We should be able to do the math. Lets make the following assumptions:
The sun loses mass spherically, and with purely radial velocity.
The earth experiences drag, due to the density times time of this gas , and the square of the earths velocity, and the cross section of the planets interaction with this gas(plasma). For a given flux of solar material passing through the surface of a sphere at the earths orbit, the density of this fluid will be inversely proportional to its radial velocity.
The numbers should be pretty uncontroversial. Some fraction, say 50% of the suns, mass, and the velocity of the earths orbit. Since we are talking about the net (drag) impulse, the rate of outflow doesn't matter. The one number I think I don't know is the cross section of the earth to drag from the solar wind plasma. Because the plasma, if electrically conductive and contains magnetic field, and the earth has a magnetic field, I would presume this effective cross section is quite a bit larger than the cross section of the solid planet. But it probably depends upon the velocity/density, and magnetic field in the solar wind -as well as the strength of the earths magnetic field.
I presume the astronomers have run the numbers, and the net drag impulse is small.
Thanks for that last answer, Ethan. I have a further question, though: why does it all clump together? (It's not just lithium, of course; this goes for other elements too). Why do we get "bodies" of ore, and not just lithium atoms mixed with gold atoms mixed with lead atoms mixed with .. well, you get the idea?
(I realise that we're at the intersection of astrophysics and geology here, but I thought I'd ask anyway).
@Ron Murray #3: That's a good question, but it's really a chemistry question, not astrophysics :-) The answer is that metallic elements vary widely in both their solubility (whether, and how much, they form ions which can be carried around by water) and in their general binding (some metals will naturally alloy with other metals, and some will only bind up with themselves).
You get ore bodies from metals which are (a) soluble at high temperatures, and (b) are non-alloying. The non-alloying part means that the metals, or maybe oxides of them, are emplaced in deep rocks as "inclusions", rather than mixed uniformly with the other metals. The high-temperature solubility means that they can be picked up by water flowing through cracks and pores deep in the crust, and carried along/upward to places where the water collects at lower temperatures. At that point the metals precipitate out of solution.
The net result is that you end up with veins of gold or silver or whatever, filling in the cracks of the country rock where water used to flow.
Welcome Ethan Siegel.
A question :
These reactions can be explained by "lithium problem"?
7Li(p,y)8Be*(a*,y)12C*
7Li(p,y)8Be*(8Be*,y)16O*
Evidence, overplus of oxygen, 16O / 12C=2 ratio?
Thank you in advance for your reply.
Regards nyemi.