Optics
In the comments to yesterday's grumpy post about the Fermi paradox, makeinu raises the idea that advanced aliens would be using more targeted communications than we do:
On the point about electromagnetic communications: even we are now using lasers to target communications with space, because it’s simply more efficient and reliable.
It’s also basically impossible to intercept, since you literally have to interrupt the beam to do so.
This is true, to a point, but when you're talking about interstellar distances, it's not quite true that you have to interrupt the beam to detect communications…
Another Monday, another recap of a new episode of the Cosmos reboot. This one was all about optics, and much of it was excellent. This was in part due to the fact that its first couple of historical segments focused on non-Western figures, and I don't know as much about their background to be able to nitpick. First up was Mozi, a Chinese philosopher from circa 400BCE, who may have been the first to demonstrate the camera obscura technique of projecting images from a pinhole in the wall of a dark room. He was followed by ibn al-Haytham, circa 1000CE, who did the first fairly complete analysis…
This year's "Flame Challenge" asks scientists to explain color in terms an 11-year-old can understand. The rules limit answers to either 300 words of text or a 6-minute video. 300 words is ridiculously short, so video is clearly the way to go. Of course, I'm not much of a video expert, but then, one of the finalists last year (when the question was "What Is Time?") was just a guy talking into a webcam, and hell, I can do better than that. So I did this:
(This is, obviously, why I was fooling around with looking at the spectrum of light from my laptop a little while back...)
The approximate…
In comments to the post on computer display colors, Will Slaton notes that Mac displays emit polarized light. And, indeed, this is an inherent part of the backlit LCD technology-- the individual pixels are bits of liquid crystal between two polarizers, and an applied voltage causes the liquid crystal molecules to flip between a state where they rotate the polarization of light, and a state where they don't. In one of those configurations, the polarizers block the light, and in the other, they let it pass. By rapidly varying the voltages, you can make the pixels flash on and off in the right…
This year's "Flame Challenge" is to explain color in terms an 11-year-old can follow. I have opinions on this subject, a background in AMO physics, and access to scientific equipment, so I'm putting something together. In the course of this, though, it occurred to me to wonder how my different portable computing devices process color. And since I have access to an Ocean Optics USB4000 spectrometer, I can answer this question in more detail than anybody needs.
So, I have three principal electronic devices that I use to do computer-type things: a Moto X smartphone, an iPad, and a Lenovo…
I'm teaching Quantum Optics this term, and one of my students picked "Atom Optics" off the list of suggested paper topics. When he asked for pointers, I said "You should check out the diffraction stuff Markus Arndt's group does." And just like that, a paper from the Arndt group turns up from the Arxiv Blog...
This is apparently only recently posted to the arxiv, though the article in Physical Chemistry Chemical Physics claims to have been online since July. Since I never get tired of talking about this, let's talk about this one, too.
So, what's this one about, then? In a lot of ways, it's…
Having spent a bunch of time talking about heavy stuff in the science blogging community, let's unwind a bit and kick the week off with a look back at an old Master's thesis. This one is from 1932, and is almost certainly a draft copy, because it's extremely cheaply bound in cardboard with the title hand-lettered on the front. There are a few corrections in the text as well, which is very short-- just 11 pages, not counting the figures at the end.
Again, this is interesting as much for what it doesn't contain as what it does. The subject matter is pretty mundane by modern standards-- just…
Yes, that's another TED@NYC picture as the "featured image," but don't run away! It's a post about science, I swear!
The photo up above is from the Flickr set (which, by the way, has been edited significantly since yesterday...), and I like it a good deal. Mostly because, as the joking caption suggests, that photo of Max Planck looming over my head has a kind of serial killer vibe to it. But here's the thing: this is the original phtoo that's on the slide:
Max Planck around the time he solved the black-body radiation problem. From wikimedia.
It's a black-and-white photo from 1901, and in…
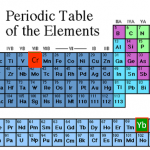
Element: Ytterbium (Yb)
Atomic Number: 70
Mass: Seven "stable" isotopes, from 168 to 176 amu. Two of those are nominally radioactive, with half-lives vastly in excess of the age of the universe.
Laser cooling wavelength: 399 nm and 556 nm.
Doppler cooling limit: 690 μK in the UV and 4.4 μK in the green.
Chemical classification: A rare earth/ lanthanide, one of the hard to distinguish metals in the little island that floats off toward the bottom of the usual presentation of the periodic table, because it's too hard to wedge them in between barium and hafnium. Yet another greyish metal.
Other…
Element: Cesium (Cs)
Atomic Number: 55
Mass: One stable isotope, mass 133 amu.
Laser cooling wavelength: 854nm, but see below.
Doppler cooling limit: 125 μK.
Chemical classification: Yet another alkali metal, column I of the periodic table.
This one isn't greyish, though! It's kind of gold color. Still explodes violently in water, though.
Other properties of interest: The definition of the second in the SI system of units is in terms of the microwave transition between hyperfine ground states in Cs-- 9,192,631,770 oscillations to one second, to be precise. Has a really large scattering…
Element: Chromium (Cr)
Atomic Number: 24
Mass: Four "stable" isotopes between 50 and 54 amu. Chromium-50 is technically radioactive, with a half-life considerably longer than the age of the universe, so...
Laser cooling wavelength: 425nm, but see below.
Doppler cooling limit: 120 μK.
Chemical classification: Transition metal, smack in the middle of the periodic table. Shiny.
Other properties of interest: Has a fairly large magnetic moment in its ground state, 6 Bohr magnetons, which means it has strong magnetic interactions. For this reason, it's kind of an interesting system to study-- you…
Element: Lithium (Li)
Atomic Number: 3
Mass: Two stable isotopes, masses 6 and 7 amu
Laser cooling wavelength: 671 nm
Doppler cooling limit: 140 μK.
Chemical classification: Alkali metal, column I in the periodic table. Yet another greyish metal. We're almost done with alkalis, I promise. Less reactive than any of the others, so the explosions in water aren't very impressive.
Other properties of interest: Lithium-7 is a boson, but has a negative scattering length, meaning that BEC's of lithium-7 tend to implode unless you modify the collisional properties. Lithium-6 is a fermion, and much…
Element: Francium (Fr)
Atomic Number: 87
Mass: Numerous isotopes ranging in mass from 199 amu to 232 amu, none of them stable. The only ones laser cooled are the five between 208 amu and 212 amu, plus the one at 221 amu.
Laser cooling wavelength: 718 nm
Doppler cooling limit: 182 μK.
Chemical classification: Alkali metal, column I in the periodic table. The heaviest known alkali, it's presumably metallic in appearance, if you could ever get enough of it together to look at.
Other properties of interest: Francium has no stable isotopes, and the longest lifetime of any of its isotopes is around…
Element: Strontium (Sr)
Atomic Number: 38
Mass: Four stable isotopes, ranging from 84 to 88 amu
Laser cooling wavelength: Two different transitions are used in the laser cooling of strontium: a blue line at 461 nm that's an ordinary sort of transition, and an exceptionally narrow "intercombination" line at 689 nm.
Doppler cooling limit: 770 μK for the blue transition, below a microkelvin for the red. The Doppler limit for the red line turns out not to be all that relevant, as other factors significantly alter the cooling process.
Chemical classification: Alkaline earth, column II of the…
Element: Xenon (Xe)
Atomic Number: 54
Mass: nine "stable" isotopes, masses from 124 to 136 amu. Xenon-136 is technically radioactive, but with a half-life of a hundred billion billion years, so, you know, it's pretty much stable.
Laser cooling wavelength: 882 nm
Doppler cooling limit: 120 μK
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with anything, so poses much less danger to scientists than any of the alkalis, though it has, at times, been used as an anesthetic, and Will Happer's group at Princeton has a funny story about a student's…
Element: Helium (He)
Atomic Number: 2
Mass: two stable isotopes, 3 and 4 amu.
Laser cooling wavelength: 1083 nm
Doppler cooling limit: 38 μK
(It should be noted, though, that despite the low temperature, laser-cooled helium has a relatively high velocity-- that Doppler limit corresponds to an average velocity that's just about the same as for sodium at 240 μK. This is because temperature is a measure of kinetic energy, and helium is much, much lighter than any of the other laser-cooled elements.)
Chemical classification: Noble gas, part of column VIII of the periodic table. Doesn't react with…
Element: Rubidium (Rb)
Atomic Number: 37
Mass: two "stable" isotopes, 85 and 87 amu (rubidium-87 is technically radioactive, but it's half-life is 48 billion years, so it might as well be stable for atomic physics purposes.
Laser cooling wavelength: 780 nm
Doppler cooling limit: 140 μK
Chemical classification: Alkali metal, column I of the periodic table. Like the majority of elements, it’s a greyish metal at room temperature. Like the other alkalis, it’s highly reactive, and bursts into flame on contact with water, even more so than sodium (in general, the alkalis get more violently reactive…
At the tail end of the cold-atom toolbox series, I joked about doing a "trading card" version shortening the posts to a more web-friendly length. In idly thinking about this, though, it occurred to me that if one were going to have cold-atom trading cards, it might make more sense to have them for the atoms, rather than the techniques. And having just devoted many thousands of words to technique, I don't really feel like trying to cut those down more, but atoms...
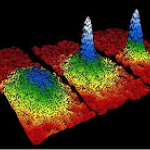
The "featured image" up top is a slide from my laser cooling lectures for our first-year seminar class. Elements outlined in red…
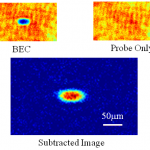
This is probably the last trip into the cold atom toolbox, unless I think of something else while I'm writing it. But don't make the mistake of assuming it's an afterthought-- far from it. In some ways, today's topic is the most important, because it covers the ways that we study the atoms once we have them trapped and cooled.
What do you mean? They're atoms, not Higgs bosons of something. You just... stick in a thermometer, or weigh them, or something... OK, actually, I have no idea. They're atoms, yes, but at ultra-low temperatures and in very small numbers. You can't bring them into…
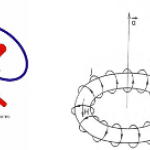
Today's dip into the cold-atom toolbox is to explain the real workhorse of cold-atom physics, the magneto-optical trap. This is the technology that really makes laser cooling useful, by letting you collect massive numbers of atoms at very low temperatures and moderate density.
Wait a minute, I thought we already had that, with optical molasses? Doesn't that make atoms really cold and stick them in space? Molasses does half the job, making the atoms really cold, but it doesn't actually confine them. The photon scattering that gives you the cooling force and Doppler cooling limit produces a "…